Abstract
Purpose
To evaluate the microvasculature alterations in convalescent Vogt-Koyanagi-Harada (VKH) disease using optical coherence tomography angiography (OCTA), and to explore the association between microvasculature and the presence of sunset glow fundus (SGF).
Methods
A cross-sectional study was conducted with 28 VKH patients at convalescent stage and 25 healthy individuals. Both eyes of each participant were enrolled. The VKH patients were classified into two subgroups based on the existence of SGF. OCTA images (3 × 3 mm) were assessed for the data of superficial capillaris plexus (SCP), deep capillaris plexus (DCP), choriocapillaris, and foveal avascular zone (FAZ).
Results
Compared with healthy control eyes and eyes without SGF, the vessel densities of the SCP and DCP decreased significantly in most regions of eyes with SGF (p < 0.0167). No significant difference of vascular perfusion was found between eyes without SGF and control eyes (p > 0.05). VKH patients with SGF had slightly increased FAZ area (p = 0.067) and decreased choroid flow area (p = 0.427) than those in the control group.
Conclusion
Convalescent VKH patients with SGF showed decreased macular capillary perfusion. OCTA could serve as a sensitive tool to assess the microvasculature alterations of VKH disease.
Similar content being viewed by others
Introuction
Vogt-Koyanagi-Harada (VKH) disease is a systemic disorder that affects eyes, meninges, auditory system and skin [1]. The main ocular manifestation is severe bilateral granulomatous posterior uveitis or panuveitis, and choroid is the main site involved [2]. VKH disease presents as four phases based on clinical features and disease progression: prodromal, acute uveitic, convalescent, and chronic recurrent. In China, VKH disease accounts for ~15.9% of all types of uveitis [3].
Optical coherence tomography angiography (OCTA) is a novel imaging tool that can provide both qualitative and quantitative information on the retinal microvasculature and choroid circulation of healthy individuals, as well as those with ocular disorders [4]. The use of OCTA in the discovery and follow-up of VKH disease may provide useful insights for doctors and patients. In a previous study, Aggarwal et al. found that the choroid ischemia manifestations demonstrated by indocyanine green angiography (ICGA) and enhanced depth imaging OCT in acute VKH patients corresponded well with the signal void detected by OCTA [5]. Moreover, the vascular density of the choriocapillaris in acute VKH patients was positively correlated with best-corrected visual acuity (BCVA), and was much lower than that of healthy controls [6]. Luo et al. identified two types of abnormalities in OCTA images obtained at acute stage and observed that the choroid flow area decreased significantly at convalescent stage compared to healthy volunteers [7].
Sunset glow fundus (SGF) is a typical sign of convalescence in VKH patients that arises due to depigmentation of choroid. Several studies have observed the variations in clinical characteristics in VKH patients with or without SGF. It has been previously demonstrated that there is a positive association between the incidence of SGF and chronic inflammation [8]. A recent study showed that eyes with higher SGF grade were positively correlated with longer disease duration and frequent recurrence [9]. Takahashi et al. reported the decreased choroidal thickness and larger peripapillary atrophy area in eyes with more severe fundus depigmentation than those in no or mild depigmentation eyes [10]. However, there have previously been no comprehensive investigations to evaluate the detailed microvasculature in convalescent VKH patients with or without fundus depigmentation. Thus, we sought to explore the association between microvasculature alterations and sunset glow fundus.
Methods
Study subjects
This cross-sectional study was performed from January 2019 to February 2020. The procedures used in this study were approved by the Ethic Committee of The Eye Hospital of Wenzhou Medical University and adhered to the statements of the Declaration of Helsinki. Informed consent was obtained from each participant. VKH was diagnosed according to the international revised diagnostic criteria [1]. The patients enrolled in this study all received oral corticosteroid with an initial dose of 1–1.2 mg/kg/day for 1–2 weeks. Following the remission of intraocular inflammation, the oral corticosteroid treatment gradually tapered. It was vital to maintain the dose of 15–20 mg/day over 4–6 months, to reduce the relapse risk [11]. To monitor potential side effects of treatment, regular examination including plasma electrolyte level, blood pressure, and blood glucose level were assessed during the follow-up period. The convalescent stage was defined as follows: disease duration >3 months, no recurrent anterior inflammation attack, and no evidence of inflammation of fundus such as serous retinal detachment or optic disc hyperemia detected by silt lamp and fundus examination. Patients and healthy controls were excluded based on the following criteria: (1) diagnosis of fundus lesion such as diabetic retinopathy, (2) presence of complications such as secondary glaucoma and secondary choroid neovascularization, (3) presence of refractive errors (spherical equivalent) >6 diopter or an axial length >26 mm, (4) signal strength index <50 or scan quality of OCTA images <6, (5) apparent projection artifacts or motion artifacts on OCTA images, and (6) a history of intraocular surgery (except for complicated cataract intervention). Detailed clinical data including age, gender, and disease duration were collected from all participants. Each patient received a comprehensive ocular examination, including BCVA, spectral-domain Optical Coherence Tomography (Spectralis, Heidelberg Engineering, Germany), OCTA (RTvue XR Avanti, Optovue Inc., Fremont, CA, USA), fundus imaging (CR-2AF, Canon Inc., Japan), and axial length measurement (IOL-Master, Carl Zeiss Inc., Germany). The diagnosis of sunset glow fundus was provided by two independent masked uveitis specialists (DL and MLD) to ensure a correct grouping and validation analysis. Any disagreement between two masked specialists was resolved by a third researcher (YQW).
OCTA imaging
Optovue Angiovue system using a spilt-spectrum amplitude-decorrelation angiography algorithm was programmed to establish the vascular structures of the retina and choroid. An A-scan rate of 70,000 scans/s and a wavelength of 840 nm were used to acquire high-resolution 3 × 3-mm images and corresponding en face images. Automatic layering was performed with software installed in the device. The superficial capillaris plexus (SCP) was defined from the inner limiting membrane to the inner plexiform layer (IPL) with 10 μm onset. The deep capillaris plexus (DCP) was defined from the IPL with 10 μm onset to outer plexiform layer (OPL) offset of 10 μm. The outer retina plexus extends from 10 μm below the OPL to 10 μm above the Bruch membrane. The choriocapillaris extends from 10 μm above the Bruch membrane to 30 μm below the Bruch membrane. All images used in this study were checked by two experienced operators to ensure their high quality and to avoid incorrect automatic segmentation by the software.
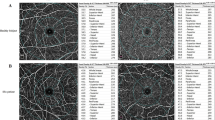
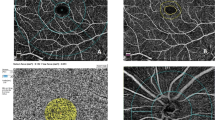
Vessel density (VD) of each region was calculated as the vessel area divided by the region area and nine regions of interest are shown in Fig. 1. The area, perimeter, and acircularity index of FAZ were also calculated automatically. The choroid flow area was analyzed in a 1-mm radius circle centered on the fovea at the level of choriocapillaris.
a Fundus photograph of the right eye from a 31-year-old man, without sunset glow fundus. f Fundus photography of the right eye from a 48-year-old man, indicating obvious sunset glow fundus. b, g Superficial vascular plexus of two representative patients. The whole image was divided into the superior and inferior region. Moreover, the foveal region was defined as a 1 mm radius circle centered on the fovea. The area between 1 mm radius and 3 mm radius circle centered on the fovea was defined as parafoveal region and the parafovea was divided into four equal sectors named temporal, superior, nasal, and inferior quadrant. c, h Deep vascular plexus of two representative patients. d, i The foveal avascular zone of two representative patients. e, j The flow area of choroid was calculated in a 1-mm radius circle centered on the fovea at the level of choriocapillaris.
Statistical analysis
Statistical analysis was performed using SPSS22.0 software. All values were expressed as mean ± standard deviation. Cohen’s kappa coefficient analysis was performed to determine the agreement between two masked observers. Univariate ANOVA and student’s t test were used to analyze normally distributed variables, and Mann–Whitney U and Kruskal–Wallis H tests were used for variables that displayed a non-normal distribution. Categorical variables were analysed via Chi-Square test. A p value <0.05 of baseline characteristics was considered to be statistically significant. Taking the small sample size into account, the average of the OCTA data of two eyes in each patient was calculated for comparison. The statistical significance of p value of the OCTA data between three groups was adopted as <0.0167 due to Bonferroni adjustment.
Results
A total of 56 eyes of 28 convalescent VKH patients without subretinal fluid and pigment epithelial detachment were enrolled in our study and divided into two subgroups based on the diagnosis of SGF. The Cohen’s kappa coefficient of the diagnosis of SGF was 0.916. Nineteen patients were placed into the non-SGF subgroup and nine patients were placed in the SGF subgroup. Fifty eyes of 25 control individuals were recruited for comparison. As shown in Table 1, there were no statistical differences in age, BCVA and spherical equivalent among three groups. The disease duration in the SGF subgroup was longer than in the non-SGF subgroup.
The vascular perfusion of the SCP and DCP were compared between the control group and VKH subgroups. The VDs in most regions of the SCP and DCP were lower in the non-SGF subgroup than in the control group, whereas these results were not statistically significant (Table 2, pa > 0.05). Excluding temporal and inferior quadrant of the DCP and foveal region, the VDs of the SGF subgroup decreased significantly than those in the control group (Table 2, pb ≤ 0.009). The VDs of several regions (including inferior of whole image, parafovea and inferior quadrant) of the SCP in the SGF subgroup decreased significantly compared with the non-SGF subgroup, while no statistical significance was observed in remaining regions (Table 2, pc ≥ 0.024). Significantly decreased VDs in the SGF subgroup were observed in most regions of the DCP when compared with the non-SGF subgroup (Table 2, pc ≤ 0.013), except for the fovea.
The area and perimeter of FAZ increased slightly in the non-SGF and SGF subgroup in comparison with the control group, without statistical significance (Table 3, p all >0.05). No significant difference of the acircularity index of FAZ and choroid flow area were determined between three groups (Table 3, p all >0.05).
Discussion
In this study, we found that the retinal VDs in most regions decreased significantly in eyes with SGF than in the control eyes. Compared with controls, a non-significant trend in the direction of lower VDs at the fovea for non-SGF patients was observed. These data may indicate that the retinal vasculature detected by OCTA is relatively well protected in eyes that do not display significant fundus depigmentation.
Although there have been many attempts to uncover the mechanisms underlying VKH disease, identifying the detailed pathogenesis remains a persistent challenge [12]. Clinical, histopathological and immunological investigations have revealed the autoimmune response against melanocytes plays a pivotal role in the development of VKH disease [2]. OCTA is an emerging technology that can be used to analyze fundus vascular structure, and provides results that are in agreement with those obtained from traditional methods such as fluorescein angiography and ICGA. Compared to traditional methods, OCTA has superior diagnostic capabilities and is able to uncover minimal amounts of damage. Therefore, using OCTA to perform comprehensive investigations in VKH may help to understand the pathogenesis of this disease. Chinese researchers have found that VDs in the foveal superficial layer and parafoveal deep layer of convalescent VKH patients decrease significantly compared with those in healthy controls. The data indicate that damage of the vascular structure can be detected in convalescent VKH patients [7].
The presence of SGF correlates with longer disease duration, as reported by previous investigations, in line with our results [8, 10]. However, the critical factors resulting in the appearance of SGF remain unknown. More severe disease and adequate systematic treatment at onset may affect the presence of SGF. Kenio et al. have demonstrated that 67.5% VKH patients treated with initial high-dose corticosteroid still develop into SGF [8]. The results in this study are, in accordance with Yang et al., that sunset glow fundus is observed in almost 23–30% VKH patients receiving sufficient and standard treatment [11]. Previous studies have determined that the SGF appearance is caused by the loss of choroidal melanocytes [13]. A possible hypothesis is persistent autoimmune response against melanocytes in the choroid could cause subclinical inflammation. The most obvious finding emerge from this analysis was that the macular capillary perfusion in most regions of the SCP and DCP in eyes with SGF decreased significantly compared with healthy eyes, whereas similar VDs were observed between eyes without SGF and control eyes. Abu El-Asrar et al. have confirmed retinal tissue hypoxia and decreased oxygen delivery of inner retina in eyes developed into SGF, whereas oxygen metabolism returns to normal in eyes without SGF [14]. Our results are, by and large, consistent with the observations measured by spectrophotometric retinal oximeter. Similar findings were also reported that VDs significantly decreased in inactive VKH patients compared with healthy volunteers [15, 16]. Multiple regression analysis has revealed that RPE undulation at acute stage is an independent predictor of fundus inflammation recurrence and deteriorated vision acuity [17]. Yang et al. proposed that photoreceptor dysfunction in convalescent VKH patients might occur as a result of RPE impairment [18], and we speculate that the structure and biological function of the RPE may not be completely restored at convalescent stage. A possible explanation for the results in present study is the subclinical inflammation of the choroid stroma may generate a persistent disruption on the retinal vasculature, especially in eyes developed into SGF.
Studies have demonstrated that FAZ is a highly sensitive predictor of retinal ischemia in diabetic retinopathy and retinal vein occlusion [19, 20]. The FAZ area and perimeter in eyes with SGF tended to be higher than in eyes without SGF and controls eyes, suggesting the potential macular ischemia. In accordance with previous study, no significantly decreased choroid flow area was observed in VKH patients when compared with control group [15]. It is worth mentioning to point out that accurate interpretation of choriocapillaris images presents a challenge because of automatic segmentation error and the presence of artifacts. Recently, Karaca et al. confirmed there was no significant difference of choroid flow area between inactive VKH patients and healthy controls, whereas Luo et al. demonstrated significantly decreased choroid flow area at convalescent stage [7, 15]. A possible explanation for this discrepancy might be the clinical characteristics of included patients in different studies and technical limitation of OCTA. Previous studies have reported the thickness of the sub-fovea choriocapillaris as approximately 7 μm, which is much thinner than the thickness obtained in this study through automatic machine segmentation [21]. Borrelli et al. demonstrate that swept-source OCTA (SS-OCTA) is an excellent tool for investigating the choriocapillaris due to its longer wavelength and stronger penetration [22]. The use of SS-OCTA in choriocapillaris analysis may further our understanding of the development of VKH disease.
This study has several limitations, which are described below. First, the small number of patients in each subgroup of this cross-sectional study might have limited our comparative analysis. Further long-term follow-up studies, including those focused on the macular and optic disc, should be conducted to investigate VD alterations along during disease progression. In addition, all OCTA images used here showed a part of the posterior pole fundus, 6 × 6 mm and 8 × 8 mm images may supply more detailed information of the peripheral region. Third, the grade of SGF has been found to correlate positively with disease duration and recurrence, indicating that investigation of the association between the SGF grade and retinal and choroidal VDs is critical.
In conclusion, our data demonstrate that eyes with sunset glow fundus at convalescent phase of VKH disease had decreased retinal vasculature density. Furthermore, our results indicate OCTA has the potential to be a convenient, noninvasive and quantitative tool for the timely assessment of microvasculature alterations and can be used to elucidate the mechanisms underlying the development and progression of VKH disease.
Summary
What was known before
-
The presence of sunset glow fundus correlates positively with disease duration, chronic inflammation and frequent recurrence in VKH disease.
-
Macular capillary perfusion of superficial capillaris plexus and deep capillaris plexus decreases significantly in inactive VKH disease than that in healthy controls.
What this study adds
-
Convalescent VKH patients with sunset glow fundus showed lower retinal vessel densities compared to healthy volunteers.
-
There is no statistical difference of retinal and choroidal microvasculature between healthy controls and convalescent VKH patients without sunset glow fundus.
References
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–52.
O’Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017;62:1–25.
Yang PZ, Zhang Z, Zhou HY, Li B, Huang XK, Gao Y, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30:943–8.
Pichi F, Sarraf D, Arepalli S, Lowder CY, Cunningham ET Jr., Neri P, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res. 2017;59:178–201.
Aggarwal K, Agarwal A, Mahajan S, Invernizzi A, Mandadi SKR, Singh R, et al. The role of optical coherence tomography angiography in the diagnosis and management of acute Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 2018;26:142–53.
Jia SS, Zhao C, Gong D, Chen Z, Zhang MF. Optical coherence tomography angiography of acute Vogt-Koyanagi-Harada disease. Zhonghua Yan Ke Za Zhi. 2017;53:735–9.
Luo K, Cai H, Hu Y, Jin C, Gan X, Deng Y, et al. Distinguishing microvasculature features of Vogt-Koyanagi-Harada in patients in acute and convalescent phases using optical coherence tomography angiography. Ocul Immunol Inflamm. 2020:1–7 https://doi.org/10.1080/09273948.2019.1695856.
Keino H, Goto H, Usui M. Sunset glow fundus in Vogt-Koyanagi-Harada disease with or without chronic ocular inflammation. Graefes Arch Clin Exp Ophthalmol. 2002;240:878–82.
Lee EK, Lee SY, Yu HG. A clinical grading system based on ultra-wide field retinal imaging for sunset glow fundus in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2015;253:359–68.
Takahashi H, Takase H, Ishizuka A, Miyanaga M, Kawaguchi T, Ohno-Matsui K, et al. Choroidal thickness in convalescent vogt-koyanagi-harada disease. Retina 2014;34:775–80.
Yang P, Ye Z, Du L, Zhou Q, Qi J, Liang L, et al. Novel treatment regimen of Vogt-Koyanagi-Harada disease with a reduced dose of corticosteroids combined with immunosuppressive agents. Curr Eye Res. 2018;43:254–61.
Dai M-L, Huang X-F, Wang Q-F, Cai W-J, Jin Z-B, Wang Y. CFI-rs7356506 polymorphisms associated with Vogt-Koyanagi-Harada syndrome. Mol Vis. 2016;22:9–17.
Inomata H, Rao NA. Depigmented atrophic lesions in sunset glow fundi of Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131:607–14.
Abu El-Asrar AM, AlBloushi AF, Gikandi PW, Hardarson SH, Stefánsson E. Retinal vessel oxygen saturation is affected in uveitis associated with Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2019;103:1695–9.
Karaca I, Yılmaz SG, Afrashi F, Nalçacı S. Assessment of macular capillary perfusion in patients with inactive Vogt-Koyanagi-Harada disease: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2020;258:1181–90.
Liang A, Zhao C, Jia S, Gao F, Han X, Pei M, et al. Retinal microcirculation defects on OCTA correlate with active inflammation and vision in Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 2020:1–7 https://doi.org/10.1080/09273948.2020.1751212.
Hashizume K, Imamura Y, Fujiwara T, Machida S, Ishida M, Kurosaka D. Retinal pigment epithelium undulations in acute stage of Vogt-Koyanagi-Harada disease: biomarker for functional outcomes after high-dose steroid therapy. Retina. 2016;36:415–21.
Yang P, Fang W, Wang L, Wen F, Wu W, Kijlstra A. Study of macular function by multifocal electroretinography in patients with Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 2008;146:767–71.
Sim DA, Keane PA, Zarranz-Ventura J, Bunce CV, Fruttiger M, Patel PJ, et al. Predictive factors for the progression of diabetic macular ischemia. Am J Ophthalmol. 2013;156:684–92.
Ghasemi Falavarjani K, Iafe NA, Hubschman J-P, Tsui I, Sadda SR, Sarraf D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after Anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Investig Ophthalmol Vis Sci. 2017;58:30–34.
Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55.
Borrelli E, Shi Y, Uji A, Balasubramanian S, Nassisi M, Sarraf D, et al. Topographic analysis of the choriocapillaris in intermediate age-related macular degeneration. Am J Ophthalmol. 2018;196:34–43.
Acknowledgements
We would like to thank all of the donors that participated in the present study. This study was supported by the National Students’ Innovation Training Program (201810343005) and XinMiao Talents Program of Zhejiang Province (2018R413036).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, S., Lin, D., Hu, J. et al. Evaluation of microvasculature alterations in convalescent Vogt-Koyanagi-Harada disease using optical coherence tomography angiography. Eye 35, 1993–1998 (2021). https://doi.org/10.1038/s41433-020-01210-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01210-5
This article is cited by
-
Bibliometric analysis of the Vogt‒Koyanagi‒Harada disease literature
International Ophthalmology (2023)
-
Decrease of choriocapillary vascular density measured by optical coherence tomography angiography in Vogt-Koyanagi-Harada disease
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)