Abstract
Background
To determine whether there is an asymmetry in bilateral sternocleidomastoid muscle (SCM) thickness in patients with unilateral congenital superior oblique palsy (SOP) and its association with surgical results.
Methods
The medical records of 186 patients with head tilt secondary to unilateral SOP, who were evaluated for the status of the SCM with neck ultrasound or magnetic resonance imaging, were reviewed. The SCM asymmetry index was calculated as a bilateral difference in the maximal muscle thickness divided by each tilted-side SCM thickness. The presence of SCM asymmetry, defined as an index of >10%, and its relationship to residual torticollis ≥5° after SOP surgery were assessed.
Results
Of 186 patients with a median age of 1.2 years, SCM asymmetry was present in 102 (54.8%) patients (6.8 ± 1.9 mm for the SOP side vs. 6.6 ± 2.1 mm for the tilted side). The SCM asymmetry did not differ according to age, amount of head tilt or hypertropia. In the patients with SCM asymmetry, more patients (87.3%) underwent physiotherapy than those without asymmetry (61.9%) (P = 0.021). In 99 patients who underwent surgery for SOP, the resolution of torticollis was not significantly different between patients with and without SCM asymmetry (87.2% vs. 76.9%, P = 0.184).
Conclusions
Nearly half of the patients with congenital SOP had SCM thickness asymmetry that was already determined at a young age. However, the surgical results did not differ significantly with respect to SCM asymmetry when physiotherapy was combined. Thus, SOP surgery can be considered despite preoperative SCM asymmetry.
Similar content being viewed by others
Introduction
Ocular torticollis is one of the most common non-muscular torticollis in children, and correcting the ophthalmic causes can improve head tilt [1, 2]. Superior oblique palsy (SOP) is one of the most common and treatable ophthalmic cause of torticollis [2,3,4,5,6,7]. However, even after SOP surgery, a third of patients have residual torticollis, which is associated with older age and sternocleidomastoid muscle (SCM) tightness [2, 4]. Thus, early surgical intervention for SOP has been advocated to prevent further musculoskeletal changes in the face and neck [4, 8, 9]. It has been reported that facial symmetry can be caused by SOP, and the presence of facial asymmetry can confirm chronicity and prevent unnecessary neurologic evaluation of SOP patients [9]. The SCM is the largest and most superficial cervical muscle, and the primary actions of the muscle are rotation of the head to the opposite side and flexion of the neck. Pathologic shortening of the SCM is the most common cause of cervical torticollis, known as congenital muscular torticollis (CMT) [2]. To the best of our knowledge, there has been no quantitative study of the SCM involved in head tilt in patients with SOP, despite the importance of the SCM in childhood torticollis. Therefore, we evaluated the bilateral difference in the maximum thickness of the SCM in patients diagnosed with unilateral congenital SOP and the association between SCM asymmetry and the surgical results of SOP.
Materials and methods
The medical records of patients who presented with a head tilt and were identified as ocular torticollis with a diagnosis of SOP at the Clinic for Torticollis of Ajou University Hospital between December 2010 and June 2019 were retrospectively reviewed. SOP was clinically diagnosed by orthoptic findings including a positive Bielschowsky head tilt test, underdepression and/or overelevation in adduction on the affected eye after comprehensive eye examinations [10, 11]. Congenital SOP was presumed based on a history of ocular torticollis from infancy, evidence of longstanding strabismus, in the case of adult patients, large vertical fusional amplitudes, and absence of torsional diplopia [10, 11]. Patients with bilateral SOP were excluded because they adopted a chin down position or changed the direction of the head tilt. Patients with any pathologic findings in the SCM, including CMT that required orthopaedic surgery, were also excluded. In addition, patients who suffered from any neurological deficit, including craniosynostosis or developmental delay, other ocular diseases including nystagmus, mechanical or restrictive strabismus, A-V pattern strabismus, combined significant horizontal deviation (exotropia > 15 prism dioptres [PD] or esotropia > 4 PD), dissociated vertical deviation and a previous history of strabismus surgery were also excluded. All patients who underwent surgery for SOP had an inferior oblique myectomy, and if the hypertropia in the primary position was >20 PD, the contralateral inferior rectus (IR) recession or ipsilateral superior rectus (SR) recession was combined [11]. The IR was recessed if the hypertropia was increased in the side contralateral to the affected eye, while the SR was recessed if the hypertropia was worse in the side ipsilateral to the affected eye [5]. In addition, the ipsilateral lateral rectus recession was performed complementarily if there was intermittent exotropia of 10–14 PD. For a significant residual hypertropia of >5 PD after the first surgery, further extraocular muscle surgeries, such as SR recession or IR recession, were additionally performed to eliminate the ocular cause of residual torticollis [5, 11]. The second surgery was conducted at least 6 months after the first surgery. Surgical success was defined as hypertropia <3 PD in the primary position and head tilt <5° at the last follow-up at least 6 months after the last surgery.
An orthopaedic assessment was performed by a co-author (SY) who was masked from the ophthalmic findings, before the eye examination. If there was a restriction in neck movement or abnormal findings, including a neck mass or hyperechoic or hyper-intense lesions suggesting fibrosis, were observed on imaging, the case was excluded because of the possibility of CMT [12]. The maximum thickness of the SCM was measured with neck ultrasound or magnetic resonance imaging (MRI). For ultrasonography of the SCM, patients were examined in the supine position, with a slight extension of the neck by gentle rotation of the head to the opposite side. For MRI of the SCM, patients were positioned in a straight supine position. The maximum thickness of the SCM was measured on the longitudinal view for the neck ultrasound and on the axial scans for the neck MRI after examining the whole length of the SCM. Measurements were performed using Picture Archiving and Communication system software (INFINITT, Seoul, Korea), which provides automatic length measurement. The SCM muscle thickness was measured twice for each patient, and the average was recorded in millimetres (mm).
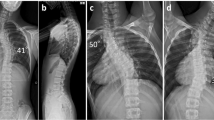
The angles of head tilt and facial asymmetry were measured on the frontal full-face photographs by a co-author (AP) who was masked from the ophthalmic and orthopaedic findings, using a toriCAM smartphone application from the App store (Apple Inc., Cupertino, CA, USA). The frontal photographs were obtained at a distance of 1 m from the patients who looked at a distant fixation target at 5 m. The degree of head tilt was measured as the difference between the midline and the median line passing through the nose of the face (Fig. 1a, b). The significant head tilt was defined as a case with head tilt of 10° or more based on the study by Lau et al. [4]. The degree of facial angle was measured as the intersection of the inter-palpebral line passing through the lateral palpebral canthi of each eye and the inter-labial line passing through the right and left angles of the mouth (Fig. 1c, d). Facial asymmetry was defined as a facial angle of 3° or more based on the 1.96 standard deviation of measurements of a previous facial morphometric study [13].
a Original frontal photograph. b The degree of head tilt measured as the difference between the midline and the median line passing through the nose of the face, showing 25° of leftward head tilt. The facial angle is calculated as the intersection of c the inter-palpebral line and d the inter-labial line, showing 4°. Parental informed consent was obtained for publication of this figure.
We used the SCM asymmetry index to describe neck muscle asymmetry independent of muscle size. The SCM asymmetry index was determined using the following equation: SCM asymmetry index (%) = (SCM tilted − SCM opposed) ÷ SCM tilted × 100. A positive value indicated that the SCM was thicker in the head-tilted side, while a negative value indicated that the SCM was thinner in the head-tilted side. SCM asymmetry was defined as an SCM asymmetry index >10% difference in bilateral SCM thickness based on defining the imbalance or torque asymmetry of bilateral muscles by a difference of more than 10% in sport science [14]. The presence and degree of SCM asymmetry were analysed according to age, direction and angle of head tilt, and amount of hypertropia before and after SOP surgery.
The protocol for this study was approved by the Institutional Review Board of Ajou University Hospital, and the need for informed consent was waived. The conduct of the study adhered to the tenets of the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 25.0 (IBM Corp, Armonk, New York, USA). Comparisons of continuous variables across the two groups were tested with the independent t-test for parametric data and the Mann–Whitney U test for nonparametric data. Categorical variables were compared using the Chi-square test or the Fisher’s exact test. A P value < 0.05 was considered statistically significant.
Results
A total of 186 patients with unilateral congenital SOP were evaluated at a median age of 1.2 years (range, 4 months to 38.3 years) for orthopaedic assessment and at a median age of 2.7 years (range, 8 months to 38.3 years) for ophthalmic examination. In total, 134 (72.0%) patients were under 2 years of age when completing the orthopaedic assessment, and there were 122 (65.6%) men and 64 (34.4%) women. Of these, 102 (54.8%) patients had right SOP and 84 (45.2%) had left SOP. All patients showed a compensatory head tilt, and three patients had a paradoxical ipsilateral head tilt. The amount of head tilt and hypertropia ranged from 3° to 38° (mean, 13.4° ± 6.0°) and 2–35 PD (mean, 7.6 ± 5.1 PD), respectively. A total of 25 (13.4%) patients also had an intermittent exotropia of <15 PD, and 146 (78.5%) patients had facial asymmetry based on facial angle of >3°. SCM thickness was measured using neck ultrasound in 154 (82.8%) patients and neck MRI in 32 (17.2%) patients. The cervical spine X-ray films were normal for all patients. A total of 141 (75.8%) patients had undergone periods of physiotherapy for SCM asymmetry or to prevent secondary musculoskeletal changes.
Of the 186 patients with SOP, SCM asymmetry was present in 102 (54.8%) patients (mean SCM thickness: 6.8 ± 1.9 mm for the SOP side vs. 6.6 ± 2.1 mm for the tilted side). Of the 102 patients with SCM asymmetry, 69 (67.6%) showed thinner SCM on the head-tilted side. Table 1 provides a comparison of the characteristics between patients with and without SCM asymmetry. Patients with SCM asymmetry were examined with neck imaging at a younger age than those with symmetric SCM (mean age of 2.4 ± 4.5 years vs. 3.7 ± 7.4 years, P = 0.002 by the independent t-test, median age of 1.2 years vs. 1.1 years, P = 0.988 by the Mann–Whitney U test). However, there was no correlation between the SCM asymmetry index and age (Fig. 2). The amount of head tilt or angle of hypertropia for patients with SCM asymmetry did not differ significantly from those without SCM asymmetry. However, the proportion of patients with a mild head tilt <10° was significantly higher in the patients with SCM asymmetry than in those without asymmetry (24.5% vs. 8.3%, P = 0.016). Furthermore, there was no significant difference in mean SCM thickness or proportion of facial asymmetry between patients with and without SCM asymmetry. In the patients with SCM asymmetry, more patients (87.3%) underwent physiotherapy than those without asymmetry (61.9%) (P = 0.021). The proportion of patients examined using MRI was significantly higher in patients with SCM asymmetry than in those without asymmetry (P = 0.018). The mean SCM thickness measured with MRI was significantly thicker than those using ultrasound (8.6 ± 2.9 mm vs. 6.2 ± 1.6 mm, P = 0.001). The mean age when performing MRI was 5.4 ± 6.4 years, which was significantly older than 2.5 ± 5.8 years for ultrasound (P = 0.029).
A total of 99 patients underwent surgery for SOP at mean age of 5.4 ± 7.8 years: 47 (46.1%) of 102 patients with SCM asymmetry and 52 (61.9%) of 84 without asymmetry, more patients without SCM asymmetry underwent SOP surgery (P = 0.022). The mean postoperative follow-up period was 21.3 ± 10.7 months (range 6–52 months). Overall, the head tilt reduced from 14.0° ± 5.3° to 3.0° ± 3.2°, and the angle of hypertropia in the primary position reduced from 9.8 ± 5.5 to 0.6 ± 2.0 PD (both P < 0.001, paired t test). Success, defined as hypertropia <3 PD and head tilt <5°, was achieved in 41 (87.2%) patients with SCM asymmetry and 40 (76.9%) without SCM asymmetry, which was not significantly different (P = 0.184). Table 2 summarises the difference between the patients with resolved head tilt and those with residual torticollis. There was no significant difference in age at first surgery, type of additional surgery, preoperative amount of head tilt, preoperative angle of hypertropia, SCM thickness, proportion of facial asymmetry and history of physiotherapy between the two groups according to surgical success.
Discussion
This study investigated the SCM thickness of patients with head tilt secondary to unilateral congenital SOP. We found that 54.8% of these patients had an SCM asymmetry. Of the patients with SCM asymmetry, two-thirds of patients showed a thinner SCM on the head-tilted side. To the best of our knowledge, this is the first report to quantify the SCM thickness asymmetry of SOP patients using imaging and correlate the findings with clinical characteristics. It is also the first attempt to objectively measure the amount of head tilt and facial angle using the toriCAM application, which is both fast and easy to perform even in infants and young children.
In SOP patients, the SCM involved in head tilt has not been well investigated. In a study by Lau et al. of 32 patients with congenital SOP, eight patients (25%) showed SCM tightness in the head-tilted side on physical examination, suggesting that residual torticollis after SOP surgery is related to SCM tightness [4]. For patients with positional plagiocephaly including ocular torticollis, one previous report has investigated SCM imbalance [15]. Sixty four of 100 patients with a mean age of 6.7 months were identified as having SCM imbalance, defined as a decreased ability to actively rotate or laterally flex their head, but with a normal passive cervical range of motion [15]. Therefore, it is not surprising that more than half of the SOP patients had SCM asymmetry on imaging in the present study. In addition, the finding of thinner SCM in the head-tilted side in our patients with a median age of 1.2 years could be related to the atrophic change with age, given that majority of the SCM tumour found in patients with CMT changed to a tight fibrotic band before 2 years of age [16].
In contrast to SCM asymmetry, the facial anomalies associated with SOP have been well described in the literature [9, 11, 13, 17,18,19]. Facial asymmetries were observed in 76–77% of patients with congenital SOP in a previous study, which is consistent with our findings that 78.5% of the SOP patients had facial asymmetry based on facial angle of >3° [17, 18]. Facial hypoplasia on the tilted side, once established, may persist despite subsequent treatment [9]. Although facial asymmetry and SCM dysfunction can be found at a very young age [15, 17, 19], SCM asymmetry may differ from the facial asymmetry in terms of chronicity. In this study, there was no correlation between the SCM asymmetry index and age; this can be explained by the differences in physiology and growth pattern between a dynamic SCM muscle and a static craniofacial deformation. During upright positioning, the infants continuously strengthen and stretch their bilateral SCM muscles, which is attributable to resolution of the SCM imbalance [15]. Stevens et al. suggested that the facial hemi-hypoplasia observed in SOP patients may be the result of gravitational forces rather than compressive forces [19]. However, the SCM runs diagonally from the back of the ear to both the collarbone and the breastbone. Therefore, gravitation may affect the SCM muscle less than the facial muscle. As the cranial shape is mostly determined during the first 6 months of life, physiotherapy for deformational plagiocephaly is known to be effective until 4 months of age, which is much earlier than that of the neck muscle [15, 20, 21]. Actually, a large portion of our patients underwent physiotherapy, which may be attributable to resolution of SCM asymmetry. However, we found that bilateral SCM thickness was relatively symmetric in older patients over 30 years who had never received physiotherapy. A longitudinal study with consecutive measures of the SCM is needed to determine this relationship.
The SCM asymmetry in the SOP patients was considered unlikely to be related to residual torticollis after SOP surgery in this study, given that there was no difference in the surgical success rate between patients with and without SCM asymmetry. The success rates of congenital SOP, defined by the resolution of head tilt, have been reported as 61.5–92% [4, 5, 11]. The overall surgical success rate, including that of the second surgery, was 81.8% in this study. Lau et al. reported that one-third of the patients with significant residual torticollis had ocular causes [4]. Moreover, Kekunnaya and Isenberg reported that one-third of their patients had residual torticollis after the initial procedure, but after the additional SOP surgery, none had significant torticollis more than 5° [5]. These findings suggest that residual torticollis in patients with SOP may be mainly due to residual ocular misalignment and not SCM dysfunction. Thus, thoroughly eliminating ophthalmic causes by SOP surgeries can successfully treat ocular torticollis, even in older patients who have longstanding abnormal head posture and facial asymmetry [4, 5, 22].
Our study has several limitations. First, the cross-sectional area of the SCM would be smaller in young children than in older patients, which could overestimate the SCM asymmetry; however, we tried to adjust for this with the proportion of differences reflecting their own muscle size. Second, we only measured amount of head tilt. SOP may be associated with head tilt, face turn, chin elevation or depression, or a combination of these [4]. However, it is difficult to investigate an active head posture using a goniometer or arthrodial protractor in young children who are full of activity [23, 24]. We focused on the most obvious findings in common clinical practice that can be easily measured. In order to quantitatively and accurately measure the abnormal head posture even in young children, it was necessary to analyse it using the frontal photographs and toriCAM. In the frontal photographs, only the head tilt was able to be measured. Third, we did not measure the SCM asymmetry after SOP surgery, and since this is a retrospective study, we could not elucidate the cause and effect relationship. Finally, we used two different imaging modalities. More SCM asymmetry was found when using MRI than ultrasound, suggesting MRI is more accurate. However, ultrasound has a great advantage of being able to obtain image without sedation even in very young patients. Further study with more patients using MRI is needed. Nevertheless, this report is the first objective description of SCM status in congenital SOP.
In conclusion, nearly half of the patients with unilateral congenital SOP had SCM thickness asymmetry at an early age. However, the resolution of torticollis did not differ with SCM asymmetry. Therefore, SOP surgery can be considered despite preoperative SCM asymmetry. However, a multidisciplinary approach is still important since majority of the patients in this study have been treated with physical therapy.
Summary
What was known before
-
Early surgical intervention for SOP has been advocated to prevent further musculoskeletal changes in the face and neck.
-
Residual torticollis after SOP surgery is associated with older age and SCM tightness.
What this study adds
-
Nearly half of the patients with congenital SOP had SCM thickness asymmetry at an early age. However, the amount of SCM asymmetry was not related to age.
-
Resolution of torticollis did not differ significantly with respect to SCM asymmetry when physiotherapy was combined.
-
SOP surgery can be considered despite preoperative SCM asymmetry.
References
Ballock RT, Song KM. The prevalence of nonmuscular causes of torticollis in children. J Pediatr Orthop. 1996;16:500–4.
Nucci P, Kushner BJ, Serafino M, Orzalesi N. A multi-disciplinary study of the ocular, orthopedic, and neurologic causes of abnormal head postures in children. Am J Ophthalmol. 2005;140:65–68.
Kushner BJ. Ocular causes of abnormal head posture. Ophthalmology. 1979;86:2115–25.
Lau FH, Fan DS, Sun KK, Yu CB, Wong CY, Lam DS. Residual torticollis in patients after strabismus surgery for congenital superior oblique palsy. Br J Ophthalmol. 2009;93:1616–9.
Kekunnaya R, Isenberg SJ. Effect of strabismus surgery on torticollis caused by congenital superior oblique palsy in young children. Indian J Ophthalmol. 2014;62:322–6.
Erkan Turan K, Taylan Sekeroglu H, Koc I, Kilic M, Sanac AS. The frequency and causes of abnormal head position based on an ophthalmology clinic’s findings: is it overlooked? Eur J Ophthalmol. 2017;27:491–4.
Mitchell PR. Ocular torticollis. Trans Am Ophthalmol Soc. 1999;97:697–769.
Goodman CR, Chabner E, Guyton DL. Should early strabismus surgery be performed for ocular torticollis to prevent facial asymmetry? J Pediatr Ophthalmol Strabismus. 1995;32:162–6.
Akbari MR, Khorrami Nejad M, Askarizadeh F, Pour FF, Ranjbar Pazooki M, Moeinitabar MR. Facial asymmetry in ocular torticollis. J Curr Ophthalmol. 2015;27:4–11.
Lee JE, Yang HK, Kim JH, Hwang JM. Ocular torsion according to trochlear nerve absence in unilateral superior oblique palsy. Invest Ophthalmol Vis Sci. 2017;58:5526–31.
Helveston EM, Mora JS, Lipsky SN, Plager DA, Ellis FD, Sprunger DT, et al. Surgical treatment of superior oblique palsy. Trans Am Ophthalmol Soc. 1996;94:315–28.
Han MH, Kang JY, Do HJ, Park HS, Noh HJ, Cho YH, et al. Comparison of clinical findings of congenital muscular torticollis between patients with and without sternocleidomastoid lesions as determined by ultrasonography. J Pediatr Orthop. 2019;39:226–31.
Velez FG, Clark RA, Demer JL. Facial asymmetry in superior oblique muscle palsy and pulley heterotopy. J AAPOS. 2000;4:233–9.
Kirkendall DT. The applied sports science of soccer. Phys Sportsmed. 1985;13:53–59.
Golden KA, Beals SP, Littlefield TR, Pomatto JK. Sternocleidomastoid imbalance versus congenital muscular torticollis: their relationship to positional plagiocephaly. Cleft Palate Craniofac J. 1999;36:256–61.
Cheng JC, Tang SP. Outcome of surgical treatment of congenital muscular torticollis. Clin Orthop Relat Res. 1999;362:190–200.
Wilson ME, Hoxie J. Facial asymmetry in superior oblique muscle palsy. J Pediatr Ophthalmol Strabismus. 1993;30:315–8.
Paysee EA, Coats DK, Plager DA. Facial asymmetry and tendon laxity in superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1995;32:158–61.
Stevens P, Downey C, Boyd V, Cole P, Stal S, Edmond J, et al. Deformational plagiocephaly associated with ocular torticollis: a clinical study and literature review. J Craniofac Surg. 2007;18:399–405.
Kluba S, Kraut W, Reinert S, Krimmel M. What is the optimal time to start helmet therapy in positional plagiocephaly? Plast Reconstr Surg. 2011;128:492–8.
Moss SD. Nonsurgical, nonorthotic treatment of occipital plagiocephaly: what is the natural history of the misshapen neonatal head? J Neurosurg. 1997;87:667–70.
Ahn SJ, Choi J, Kim SJ, Yu YS. Superior rectus muscle recession for residual head tilt after inferior oblique muscle weakening in superior oblique palsy. Korean J Ophthalmol. 2012;26:285–9.
Ohman AM, Beckung ER. Reference values for range of motion and muscle function of the neck in infants. Pediatr Phys Ther. 2008;20:53–58.
Rahlin M, Sarmiento B. Reliability of still photography measuring habitual head deviation from midline in infants with congenital muscular torticollis. Pediatr Phys Ther. 2010;22:399–406.
Funding
This study was supported by the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (NRF-2017R1C1B5017453; Seoul, South Korea).
Author information
Authors and Affiliations
Contributions
Study concept and design (SAC, S-YY); data collection (SAC, AP, S-YY); analysis and interpretation of data (SAC, S-YY); drafting of the manuscript (SAC, S-YY); critical revision of the manuscript (SAC, AP, S-YY); statistical expertise (SAC, S-YY) and supervision (SAC, S-YY).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was conducted in accordance with the tenets of the Declaration of Helsinki; the research protocol was approved by the Institutional Review Board of Ajou University School of Medicine, Suwon, South Korea (AJIRB-MED-OBS-20-096).
Informed consent
The need for informed consent was waived because this was a retrospective observational study using pre-existing medical records and imaging.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, S.A., Yim, SY. & Park, A. Sternocleidomastoid muscle asymmetry in unilateral congenital superior oblique palsy. Eye 35, 1954–1960 (2021). https://doi.org/10.1038/s41433-020-01205-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01205-2