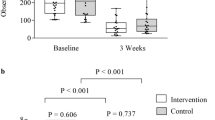
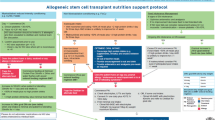
Abstract
Nutrition support is often required during hematopoietic cell transplant (HCT) given the gastrointestinal toxicity that frequently precludes adequate protein-calorie intake. This article reviews the latest evidence for enteral versus parenteral nutrition in the adult and pediatric HCT population and addresses key considerations as well as barriers to implement this in practice. Registered Dietitian Nutritionists are key members of the interdisciplinary team to proactively manage enteral nutrition support to provide timely, adequate protein and calories to help prevent malnutrition, loss of lean body mass, and functional decline as well as provide evidence-based diet recommendations. This article also reviews emerging research supporting the role of luminal nutrients to maintain microbiotal diversity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Iestra JA, Fibbe WE, Zwinderman AH, Romijn JA, Kromhout D. Parenteral nutrition following intensive cytotoxic therapy: an exploratory study on the need for parenteral nutrition after various treatment approaches for haematological malignancies. Bone Marrow Transpl. 1999;23:933–9. https://doi.org/10.1038/sj.bmt.1701747.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. https://doi.org/10.1016/j.clnu.2016.07.015.
Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr. 2019;38:738–44. https://doi.org/10.1016/j.clnu.2018.03.008.
Clemmons AB, Orr J, Andrick B, Gandhi A, Sportes C, DeRemer D. Randomized, placebo-controlled, phase III trial of Fosaprepitant, Ondansetron, Dexamethasone (FOND) versus FOND Plus Olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: the FOND-O trial. Biol Blood Marrow Transpl. 2018;24:2065–71. https://doi.org/10.1016/j.bbmt.2018.06.005.
Pastore D, Bruno B, Carluccio P, Stella De Candia M, Mammoliti S, Borghero C, et al. Antiemetic prophylaxis in patients undergoing hematopoietic stem cell transplantation: a multicenter survey of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) transplant programs. Ann Hematol. 2020;99:867–75. https://doi.org/10.1007/s00277-020-03945-3.
Walrath M, Bacon C, Foley S, Fung HC. Gastrointestinal side effects and adequacy of enteral intake in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2015;30:305–10. https://doi.org/10.1177/0884533614547084.
Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–8. https://doi.org/10.1182/blood-2004-03-0804.
Topcuoglu P, Arat M, Ozcan M, Arslan O, Ilhan O, Beksac M, et al. Case-matched comparison with standard versus reduced intensity conditioning regimen in chronic myeloid leukemia patients. Ann Hematol. 2012;91:577–86. https://doi.org/10.1007/s00277-011-1349-2.
Andersen S, Brown T, Kennedy G, Banks M. Implementation of an evidenced based nutrition support pathway for haematopoietic progenitor cell transplant patients. Clin Nutr. 2015;34:536–40. https://doi.org/10.1016/j.clnu.2014.06.006.
Doney K, McMillen K, Buono L, Deeg HJ, Gooley T. Impact of body mass index on outcomes of hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transpl. 2019;25:613–20. https://doi.org/10.1016/j.bbmt.2018.10.006.
Deeg HJ, Seidel K, Bruemmer B, Pepe MS, Appelbaum FR. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transpl. 1995;15:461–8.
Andersen S, Weber N, Kennedy G, Brown T, Banks M, Bauer J. Tolerability of proactive enteral nutrition post allogeneic haematopoietic progenitor cell transplant: a randomised comparison to standard care. Clin Nutr. 2020;39:1364–70. https://doi.org/10.1016/j.clnu.2019.06.012.
Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–53. https://doi.org/10.1182/blood-2014-02-514778.
Baumgartner A, Bargetzi A, Zueger N, Bargetzi M, Medinger M, Bounoure L, et al. Revisiting nutritional support for allogeneic hematologic stem cell transplantation-a systematic review. Bone Marrow Transpl. 2017;52:506–13. https://doi.org/10.1038/bmt.2016.310.
Uderzo C, Rovelli A, Bonomi M, Fomia L, Pirovano L, Masera G. Total parenteral nutrition and nutritional assessment and leukaemic children undergoing bone marrow transplantation. Eur J Cancer. 1991;27:758–62. https://doi.org/10.1016/0277-5379(91)90183-e.
Yokoyama S, Fujimoto T, Mitomi T, Yabe M, Yabe H, Kato S. Use of total parenteral nutrition in pediatric bone marrow transplantation. Nutrition. 1989;5:27–30.
Peric Z, Botti S, Stringer J, Krawczyk J, et al. Variability of nutritional practices in peritransplant period after allogeneic hematopoietic stem cell transplantation: a survey by the Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transpl. 2018;53:1030–7. https://doi.org/10.1038/s41409-018-0137-1.
Fuji S, Einsele H, Savani BN, Kapp M. Systematic nutritional support in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transpl. 2015;21:1707–13. https://doi.org/10.1016/j.bbmt.2015.07.003.
Andersen S, Banks M, Brown T, Weber N, Kennedy G, Bauer J. Nutrition support during allogeneic stem cell transplantation: evidence versus practice. Support Care Cancer. Published online March 10, 2020. https://doi.org/10.1007/s00520-020-05397-x.
Staffas A, Burgos da Silva M, van den Brink MRM. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129:927–33. https://doi.org/10.1182/blood-2016-09-691394.
Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transpl. 2015;21:1373–83. https://doi.org/10.1016/j.bbmt.2015.04.016.
D’Amico F, Biagi E, Rampelli S, Fiori J, Zama D, Soverini M, et al. Enteral nutrition in pediatric patients undergoing hematopoietic SCT promotes the recovery of gut microbiome homeostasis. Nutrients. 2019;11. https://doi.org/10.3390/nu11122958.
White M, Murphy AJ, Hallahan A, Ware RS, Fraser C, Davies PSW. Survival in overweight and underweight children undergoing hematopoietic stem cell transplantation. Eur J Clin Nutr. 2012;66:1120–3. https://doi.org/10.1038/ejcn.2012.109.
Hoffmeister PA, Storer BE, Macris PC, Carpenter PA, Baker KS. Relationship of body mass index and arm anthropometry to outcomes after pediatric allogeneic hematopoietic cell transplantation for hematologic malignancies. Biol Blood Marrow Transpl. 2013;19:1081–6. https://doi.org/10.1016/j.bbmt.2013.04.017.
Lipkin AC, Lenssen P, Dickson BJ. Nutrition issues in hematopoietic stem cell transplantation: state of the art. Nutr Clin Pract. 2005;20:423–39. https://doi.org/10.1177/0115426505020004423.
Baumgartner A, Zueger N, Bargetzi A, Medinger M, Passweg JR, Stanga Z, et al. Association of Nutritional Parameters with Clinical Outcomes in patients with acute myeloid leukemia undergoing haematopoietic stem cell transplantation. Ann Nutr Metab. 2016;69:89–98. https://doi.org/10.1159/000449451.
Horsley P, Bauer J, Gallagher B. Poor nutritional status prior to peripheral blood stem cell transplantation is associated with increased length of hospital stay. Bone Marrow Transpl. 2005;35:1113–6. https://doi.org/10.1038/sj.bmt.1704963.
August DA, Huhmann MB, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. J Parenter Enter Nutr. 2009;33:472–500. https://doi.org/10.1177/0148607109341804.
Thompson JL, Duffy J. Nutrition support challenges in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2008;23:533–46. https://doi.org/10.1177/0884533608323423.
Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18:283–95. https://doi.org/10.1038/nrc.2018.10.
Halpern-Silveira D, Susin LRO, Borges LR, Paiva SI, Assunção MCF, Gonzalez MC. Body weight and fat-free mass changes in a cohort of patients receiving chemotherapy. Support Care Cancer. 2010;18:617–25. https://doi.org/10.1007/s00520-009-0703-6.
Ferguson ML, Bauer J, Gallagher B, Capra S, Christie DR, Mason BR. Validation of a malnutrition screening tool for patients receiving radiotherapy. Australas Radio. 1999;43:325–7. https://doi.org/10.1046/j.1440-1673.1999.433665.x.
Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458–64. https://doi.org/10.1016/s0899-9007(99)00084-2.
Isenring E, Bauer J, Capra S. The scored Patient-generated Subjective Global Assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr. 2003;57:305–9. https://doi.org/10.1038/sj.ejcn.1601552.
Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–85. https://doi.org/10.1038/sj.ejcn.1601412.
Isenring E, Cross G, Daniels L, Kellett E, Koczwara B. Validity of the malnutrition screening tool as an effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support Care Cancer. 2006;14:1152–6. https://doi.org/10.1007/s00520-006-0070-5.
Becker P, Carney LN, Corkins MR, Monczka J, Smith E, Smith SE, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract. 2015;30:147–61. https://doi.org/10.1177/0884533614557642.
White M, Murphy AJ, Hastings Y, Shergold J, Young J, Montgomery C, et al. Nutritional status and energy expenditure in children pre-bone-marrow-transplant. Bone Marrow Transpl. 2005;35:775–9. https://doi.org/10.1038/sj.bmt.1704891.
Steele C, Salazar A, Rypkema L. Utilization of a nutrition support algorithm reduces unnecessary parenteral nutrition use in pediatric oncology inpatients. J Acad Nutr Diet. 2016;116:1235–8. https://doi.org/10.1016/j.jand.2015.12.007.
Murphy AJ, White M, Viani K, Mosby TT. Evaluation of the nutrition screening tool for childhood cancer (SCAN). Clin Nutr. 2016;35:219–24. https://doi.org/10.1016/j.clnu.2015.02.009.
Co-Reyes E, Li R, Huh W, Chandra J. Malnutrition and obesity in pediatric oncology patients: causes, consequences, and interventions. Pediatr Blood Cancer. 2012;59:1160–7. https://doi.org/10.1002/pbc.24272.
Elia M, Stratton RJ. Considerations for screening tool selection and role of predictive and concurrent validity. Curr Opin Clin Nutr Metab Care. 2011;14:425–33. https://doi.org/10.1097/MCO.0b013e328348ef51.
Secker DJ, Jeejeebhoy KN. How to perform Subjective Global Nutritional assessment in children. J Acad Nutr Diet. 2012;112:424–.e6. https://doi.org/10.1016/j.jada.2011.08.039.
Bowman LC, Williams R, Sanders M, Ringwald-Smith K, Baker D, Gajjar A. Algorithm for nutritional support: experience of the Metabolic and Infusion Support Service of St. Jude Children’s Research Hospital. Int J Cancer Suppl. 1998;11:76–80.
Fuji S, Rovó A, Ohashi K, Griffith M, Einsele H, Kapp M, et al. How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT. Bone Marrow Transpl. 2016;51:1041–9. https://doi.org/10.1038/bmt.2016.81.
Sopfe J, Pyle L, Keating AK, Campbell K, Liu AK, Wadwa RP, et al. Malglycemia is associated with poor outcomes in pediatric and adolescent hematopoietic stem cell transplant patients. Blood Adv. 2019;3:350–9. https://doi.org/10.1182/bloodadvances.2018021014.
Fuji S, Kim S-W, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007;84:814–20. https://doi.org/10.1097/01.tp.0000296482.50994.1c.
Sheean PM, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2006;12:656–64. https://doi.org/10.1016/j.bbmt.2006.01.010.
Verdi Schumacher M, Moreira, Faulhaber GA. Nutritional status and hyperglycemia in the peritransplant period: a review of associations with parenteral nutrition and clinical outcomes. Rev Bras Hematol E Hemoter. 2017;39:155–62. https://doi.org/10.1016/j.bjhh.2016.09.016.
Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94:287–94. https://doi.org/10.1097/TP.0b013e3182558f60.
Gonzales F, Bruno B, Alarcón Fuentes M, De Berranger E, Guimber D, Behal H, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr. 2018;37:2113–21. https://doi.org/10.1016/j.clnu.2017.10.005.
Herrmann VM, Petruska PJ. Nutrition support in bone marrow transplant recipients. Nutr Clin Pract. 1993;8:19–27. https://doi.org/10.1177/011542659300800119.
Langdana A, Tully N, Molloy E, Bourke B, O’Meara A. Intensive enteral nutrition support in paediatric bone marrow transplantation. Bone Marrow Transpl. 2001;27:741–6. https://doi.org/10.1038/sj.bmt.1702855.
Taur Y, Jenq RR, Perales M, Littman ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–82. https://doi.org/10.1182/blood-2014-02-554725.
Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transpl. 2014;20:640–5. https://doi.org/10.1016/j.bbmt.2014.01.030.
Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11. https://doi.org/10.3390/nu11071613.
Andermann TM, Peled JU, Ho C, Reddy P, Riches M, Storb R, et al. The microbiome and hematopoietic cell transplantation: past, present, and future. Biol Blood Marrow Transpl. 2018;24:1322–40. https://doi.org/10.1016/j.bbmt.2018.02.009.
Moody K, Charlson ME, Finlay J. The neutropenic diet: what’s the evidence? J Pediatr Hematol Oncol. 2002;24:717–21. https://doi.org/10.1097/00043426-200212000-00007.
USDA Food Safety and Insepection Service. Food safety for transplant recipients. https://www.fsis.usda.gov/shared/PDF/Food_Safety_for_Transplant_Recipients.pdf.
Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382:822–34. https://doi.org/10.1056/NEJMoa1900623.
Stein-Thoeringer CK, Nichols KB, Lazrak A, Docampo MD, Slingerland AE, Slingerland JB, et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science. 2019;366:1143–9. https://doi.org/10.1126/science.aax3760.
Bafeta A, Koh M, Riveros C, Ravaud P.Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review.Ann Intern Med. 2018;169:240–7. https://doi.org/10.7326/M18-0343.
Hassan H, Rompola M, Glaser AW, Kinsey SE, Phillips RS. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. 2018;26:2503–9. https://doi.org/10.1007/s00520-018-4216-z.
Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60:S129–134. https://doi.org/10.1093/cid/civ085.
White JV, Guenter P, Jensen G, Malone A, Schofield M.Academy Malnutrition Work Group et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112:730–8. https://doi.org/10.1016/j.jand.2012.03.012.
McGrath KH, Evans V, Yap J. Indications and patterns of use for parenteral nutrition in pediatric oncology. JPEN J Parenter Enter Nutr. 2020;44:632–8. https://doi.org/10.1002/jpen.1685.
Deswarte-Wallace J, Firouzbakhsh S, Finklestein JZ. Using research to change practice: enteral feedings for pediatric oncology patients. J Pediatr Oncol Nurs. 2001;18:217–23. https://doi.org/10.1053/jpon.2001.26875.
Guièze R, Lemal R, Cabrespine A, Hermet E, Tournilhac O, Combal C, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33:533–8. https://doi.org/10.1016/j.clnu.2013.07.012.
Mueller C. The ASPEN Adult Nutrition Support Core Curriculum; 2017. Accessed 5 Sep 2020. http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=1831579.
Juvé-Udina M-E, Valls-Miró C, Carreño-Granero A, Martinez-Estalella G, Monterde-Prat D, Domingo-Felici C, et al. To return or to discard? Randomised trial on gastric residual volume management. Intensive Crit Care Nurs. 2009;25:258–67. https://doi.org/10.1016/j.iccn.2009.06.004.
Taylor SJ, Manara AR, Brown J. Treating delayed gastric emptying in critical illness: metoclopramide, erythromycin, and bedside (cortrak) nasointestinal tube placement. J Parenter Enter Nutr. 2010;34:289–94. https://doi.org/10.1177/0148607110362533.
Patel RP, Canada TW, Nates JL. Bleeding associated with feeding tube placement in critically Ill oncology patients with thrombocytopenia. Nutr Clin Pract. 2016;31:111–5. https://doi.org/10.1177/0884533615598964.
da Silva JSV, Seres DS, Sabino K, Adams SC, Berdahl GJ, Citty SW, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. 2020;35:178–95. https://doi.org/10.1002/ncp.10474.
Charuhas Macris P, Schilling K, Palko R. Academy of Nutrition and Dietetics: revised 2017 standards of practice and standards of professional performance for registered dietitian nutritionists (competent, proficient, and expert) in oncology nutrition. J Acad Nutr Diet. 2018;118:736–.e42. https://doi.org/10.1016/j.jand.2018.01.012.
Academy Quality Management Committee. Academy of Nutrition and Dietetics: revised 2017 scope of practice for the registered dietitian nutritionist. J Acad Nutr Diet. 2018;118:141–65. https://doi.org/10.1016/j.jand.2017.10.002.
Thompson KL, Elliott L, Fuchs-Tarlovsky V, Levin RM, Voss AC, Piemonte T. Oncology evidence-based nutrition practice guideline for adults. J Acad Nutr Diet. 2017;117:297–310.e47. https://doi.org/10.1016/j.jand.2016.05.010.
Andrassy RJ, Chwals WJ. Nutritional support of the pediatric oncology patient. Nutrition. 1998;14:124–9. https://doi.org/10.1016/s0899-9007(97)00225-6.
Bauer J, Jürgens H, Frühwald MC. Important aspects of nutrition in children with cancer. Adv Nutr. 2011;2:67–77. https://doi.org/10.3945/an.110.000141.
Bendelsmith CR, Linabery AM, Nickel AJ, Laquere RM, Ingram KM, Hansen MB, et al. Effects of proactive and rescue enteral tube feedings on weight change in children undergoing treatment for high-grade CNS tumors. Neuro-Oncol Pract. 2020;7:428–38. https://doi.org/10.1093/nop/npaa003.
Hastings Y, White M, Young J. Enteral nutrition and bone marrow transplantation. J Pediatr Oncol Nurs. 2006;23:103–10. https://doi.org/10.1177/1043454205285866.
Ward EJ, Henry LM, Friend AJ, Wilkins S, Phillips RS. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database Syst Rev. 2015;CD003298. https://doi.org/10.1002/14651858.CD003298.pub3.
Evans J, Needle JJ, Hirani SP. Early outcomes of gastrostomy feeding in paediatric allogenic bone marrow transplantation: a retrospective cohort study. Clin Nutr ESPEN. 2019;31:71–79. https://doi.org/10.1016/j.clnesp.2019.02.014.
Hannah E, John RM. Everything the nurse practitioner should know about pediatric feeding tubes. J Am Assoc Nurse Pract. 2013;25:567–77. https://doi.org/10.1002/2327-6924.12075.
Azarnoush S, Bruno B, Beghin L, Guimber D, Nelken B, Yakoub-Agha I, et al. Enteral nutrition: a first option for nutritional support of children following allo-SCT? Bone Marrow Transpl. 2012;47:1191–5. https://doi.org/10.1038/bmt.2011.248.
DeMille D, Deming P, Lupinacci P, Jacobs LA. The effect of the neutropenic diet in the outpatient setting: a pilot study. Oncol Nurs Forum. 2006;33:337–43. https://doi.org/10.1188/ONF.06.337-343.
Moody K, Finlay J, Mancuso C, Charlson M. Feasibility and safety of a pilot randomized trial of infection rate: neutropenic diet versus standard food safety guidelines. J Pediatr Hematol Oncol. 2006;28:126–33. https://doi.org/10.1097/01.mph.0000210412.33630.fb.
Trifilio S, Helenowski I, Giel M, Gobel B, Pi J, Greenberg D, et al. Questioning the role of a neutropenic diet following hematopoetic stem cell transplantation. Biol Blood Marrow Transpl. 2012;18:1385–90. https://doi.org/10.1016/j.bbmt.2012.02.015.
Boyle NM, Podczervinski S, Jordan K, Stednick Z, Butler-Wu S, McMillen K, et al. Bacterial foodborne infections after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2014;20:1856–61. https://doi.org/10.1016/j.bbmt.2014.06.034.
Taggart C, Neumann N, Alonso PB, Lane A, Pate A, Stegman A, et al. Comparing a neutropenic diet to a food safety-based diet in pediatric patients undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2019;25:1382–6. https://doi.org/10.1016/j.bbmt.2019.03.017.
Sonbol MB, Jain T, Firwana B, Hilal T, Deleon T, Murad A, et al. Neutropenic diets to prevent cancer infections: updated systematic review and meta-analysis. BMJ Support Palliat Care. 2019;9:425–33. https://doi.org/10.1136/bmjspcare-2018-001742.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McMillen, K.K., Coghlin-Dickson, T. & Adintori, P.A. Optimization of nutrition support practices early after hematopoietic cell transplantation. Bone Marrow Transplant 56, 314–326 (2021). https://doi.org/10.1038/s41409-020-01078-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01078-9
This article is cited by
-
A predictive model combining clinical characteristics and nutritional risk factors for overall survival after umbilical cord blood transplantation
Stem Cell Research & Therapy (2023)
-
Antiemetic Strategies in Patients Who Undergo Hematopoietic Stem Cell Transplantation
Clinical Hematology International (2022)
-
Roles of the intestinal microbiota and microbial metabolites in acute GVHD
Experimental Hematology & Oncology (2021)