Abstract
Background
In intensive care units (ICUs), both continuous and intermittent feeding are commonly used for early enteral nutrition (EN). However, whether continuous feeding is a preferable feeding modality compared to intermittent feeding remains unclear. Therefore, this meta-analysis assessed the clinical efficacy of both EN feeding modalities in critically ill patients.
Methods
The PubMed, Embase, and Cochrane Library databases were searched from their inception dates to December 29, 2022. The search did not involve language restrictions (PROSPERO CRD42022371756). Randomized controlled trials (RCTs) comparing the clinical efficacy and safety of continuous feeding and intermittent feeding in critically ill patients in ICUs were included.
Results
We included 13 RCTs involving 785 patients. Compared with intermittent feeding, continuous feeding was associated with a lower mortality rate (relative risk [RR], 0.68; 95% confidence interval [CI], 0.47, 0.98; p = 0.04) but a higher risk of constipation (RR, 1.57; 95% CI, 1.02, 2.43; p = 0.04). Trial sequential analysis (TSA) for mortality rate presented a cumulative Z-curve crossing the traditional boundary, but the curve did not cross the TSA boundary for benefit. No significant differences were found in the aspiration/pneumonia rate (RR, 1.19; 95% CI, 0.51, 2.75; p = 0.69), diarrhea rate (RR, 0.82; 95% CI, 0.58, 1.16; p = 0.26), or increased gastric residual volumes (RR, 1.05; 95% CI, 0.58, 1.90; p = 0.86) between the groups.
Conclusion
Despite the low certainty of evidence, compared with intermittent feeding, continuous feeding may reduce the mortality rate in critically ill patients in ICU. Additional studies are needed to provide more evidence and validate the findings.
Similar content being viewed by others
Introduction
In the intensive care units (ICUs), critically ill patients are stressed by metabolic and hormonal derangements, which leads to malnutrition along with macronutrient and micronutrient deficiencies [1]. Early enteral nutrition (EN) feeding is recommended for most critically ill patients to mitigate the catabolic state and prevent intestinal villous atrophy, enterocyte apoptosis, inflammatory infiltration, dysbiosis, and gut immune function disorders [2, 3]. Multiple studies have demonstrated that the infection rate was lower in critically ill patients receiving early EN [2, 4, 5]. Although EN is an acknowledged feeding modality, the optimal EN feeding modality remains unknown.
Both continuous feeding and intermittent feeding modalities are commonly applied for critically ill patients. Continuous feeding delivers nutrients hourly via a feeding pump, whereas intermittent feeding provides a large volume of food over 20–60 min every 4–6 h through gravity assist [6]. Clinical guidelines have recommended continuous feeding for critically ill patients because of its advantages in reducing the rate of diarrhea and increasing feeding volume [2, 4].
Continuous feeding is provided throughout the day, which may lead to decreased gastrointestinal hormone secretion, insulin resistance, and hyperglycemia. By contrast, intermittent feeding is more suitable for our bodies because of the intermittent ingestion of nutrients a few times a day, which might theoretically provide metabolic and physiological benefits, making it superior to continuous feeding in critically ill patients [7, 8]. Yet, intermittent feeding is associated with higher rate of diarrhea, feeding intolerance, and aspiration pneumonia, according to a previous study [2].
Several meta-analyses [9,10,11,12] have compared the clinical efficacy and safety of continuous and intermittent feeding in ICU patients. A recent study by Heffernan et al. found an increased risk of constipation in patients receiving continuous feeding in ICUs, whereas no significant differences in other outcomes, including mortality, diarrhea, pneumonia, and increased gastric residuals, were observed [12]. However, another study by Ma et al. demonstrated that patients receiving intermittent feeding had a significantly higher occurrence of feeding intolerance than with continuous feeding [10]. Because of the heterogeneity of the findings, the clinical efficacy and safety of continuous feeding compared relative to intermittent feeding remains controversial. With new randomized controlled trials (RCTs) available, we conducted this systematic review and meta-analysis, which is the latest study composed entirely of RCTs. To verify the strength of the current conclusion, we perform additional trial sequential analysis (TSA), which were not included in the previous SRMA. Similar to the ASPEN and ESPEN guidelines, we also refer to GRADE to assess the certainty of evidence for each outcome, which enables clinical practitioners to view the results more objectively. This study aims to provide updated information and clear evidence on the effects of different enteral feeding modalities in critically ill patients.
Materials and methods
This systematic review and meta-analysis was registered in PROSPERO (registration number ID: CRD42022371756) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [13].
Inclusion criteria and exclusion criteria
We included RCTs that met the following criteria: (1) including patients aged ≥18 years; (2) including patients admitted to the ICUs with any diagnosis; (3) comparing the efficacy and safety between continuous and intermittent feeding in an ICU; and (4) applying an RCT design.
We excluded studies that met the following criteria: (1) being letters, study protocols, phase I or II studies, animal studies, redundant and unrelated publications, studies in non-ICU settings, or case reports; and (2) including literature with ineligible outcomes. These studies were excluded after reading the titles and abstracts, and the full texts of the remainder were obtained for quality assessment and data synthesis. In addition, individual journals and conference proceedings, reference lists of related studies, and systematic reviews and meta-analyses were manually examined to identify any additional publications relevant to our topic.
Search strategy
The PubMed, Embase, and Cochrane Library databases were searched from their inception dates to December 29, 2022. A combination of controlled vocabulary, free-text words and synonyms as keywords were employed, with no limitations on language or publication date. We also utilized Boolean search to ensure an exhaustive search. The search strategy is detailed in Table S1.
Study selection
Two investigators (JYW and THL) independently screened the titles and abstracts of the articles collected using the aforementioned search strategies to identify and assess potentially eligible studies. Disagreements were resolved by input from a third investigator (CCL). Full-text copies of potentially relevant articles were also obtained and reviewed for eligibility. No limitations were imposed regarding language, age, sex, race, or ethnicity.
Data extraction
We extracted the baseline characteristics and outcomes of patients in the included studies. The following information was extracted: author, publication year, study participants, number of participants, gender, age, APACHE II score, feeding program, and outcomes. The primary outcome was mortality rate. Secondary outcomes of interest were risk of aspiration/pneumonia, diarrhea, constipation, and increased gastric residual volumes (GRV). In situations with a lack of available data in the published articles, we proactively reached out to the corresponding authors to acquire the original data.
Assessment of risk of bias
The Cochrane risk of bias 2.0 tool was used to assess the risk of bias in the included RCTs [14]; this was done by two investigators (JYW and PYH). Six domains of bias were evaluated (allocation, performance, attrition, detection, reporting, and overall bias) and were coded as having a low, some, or high risk of bias.
Statistical analysis
The data were synthesized using the Mantel–Haenszel and inverse-variance-weighted random-effects models to estimate the overall pooled effect, which is expressed as relative risk (RR) with 95% confidence interval (CI). Statistical analyses were performed using Review Manager Version 5.4.1 and the “metafor” package in the R software 4.2.1. p < 0.05 was considered statistically significant, except for the statistical test for heterogeneity, where 0.1 was used.
The levels of heterogeneity among the included studies were determined using Hedge’s I2 tests. Moreover, I2 ≤ 25% was considered to indicate low heterogeneity, 25% < I2 < 75% was considered to indicate moderate heterogeneity, and I2 ≥ 75% was considered to indicate high heterogeneity [15]. As moderate to high heterogeneity was detected among the included trials, we conducted the “leave-one-out” sensitivity analysis and Baujat plot to assess the contribution of each study on the unexplained heterogeneity [15,16,17]. Funnel plot was used to examine the potential publication bias for primary outcome [18].
Trial sequential analysis
Trial sequential analysis (TSA) is a method for evaluating the statistical significance of the results of a clinical trial or meta-analysis and provides a more accurate assessment of results’ reliability [19]. For primary outcomes, we performed TSA with an overall type I error of 5%, type II error of 20%, and RR reduction of 20%. TSA was conducted by using TSA version 0.9.5.10 beta (Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark).
Certainty of evidence
The certainty of evidence for each outcome was assessed independently by two reviewers (JYW and PYH) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which has five domains (risk of bias, inconsistency, indirectness, imprecision, and publication bias). Each domain is graded as having “very low,” “low,” “moderate,” or “high certainty of evidence” [17] by using GRADEpro GDT software (Available at gradepro.org).
Results
Description of included studies
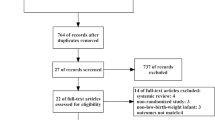
The flowchart of the literature search process is shown in Fig. 1. We identified 1368 studies in our initial search, and eight additional studies were retrieved after screening reference lists of related articles. Subsequently, a total of 13 RCTs involving 785 patients were included in this meta-analysis [20,21,22,23,24,25,26,27,28,29,30,31,32] (Fig. 1). Among the included patients, their age ranged from 35 to 69 years, and their APACHE II scores ranged from 12 to 28. Regarding the enrolled patients, patients in five studies were in the surgical ICU [23, 26, 27, 29, 30], patients in three studies were in the medical ICU [20,21,22], patients in one study were in the neurology ICU [32], and patients in four studies were in a mixed ICU [24, 25, 28, 31]. Continuous feeding was defined as delivery of EN at a constant speed for 18–24 h via a nutritional pump. Three studies mentioned an initial feeding rate of 20–25 ml/h [20, 27, 30]. Intermittent feeding was defined as the delivery of EN multiple times, generally 4–6 times/day for 60 min. Six studies mentioned bolus feeding; however, the bolus feeding technique is similar to intermittent feeding [21, 22, 24, 26, 28, 30] (Table 1).
Risk of bias assessment
The specific experimental design did not achieve the criteria of “low risk of bias” in the domains of allocation concealment and blinding, leading to “some concern” to “high risk” of overall bias in most included studies (Fig. S1). Detection bias was considered low because mortality rate is an objective outcome, which is unlikely influenced by the observer.
Primary outcome
Overall, the mortality rate of the continuous feeding group was 12.8% (event/total numbers = 35/273), which was lower than that of the intermittent feeding group (18.9% [event/total numbers = 52/275]). Seven studies involving 548 patients reported the mortality rate, which was significantly lower in patients receiving continuous feeding (RR, 0.68; 95% CI, 0.47, 0.98; p = 0.04, Fig. 2).
By performing TSA, the required sample size was 3289 patients to assess the mortality rate. TSA also revealed that the cumulative Z-curve crossed the traditional boundary, which showed the superiority of the continuous feeding group; however, it did not cross the TSA boundary for benefit, indicating that the mortality rate remains uncertain. Only 16.67% of the required information (event/total numbers = 548/3289) was accrued in the current analysis (Fig. 3). Additional studies are needed to validate the findings.
Secondary outcomes
In five studies with 228 patients, a significantly higher risk of constipation in patients receiving continuous feeding was observed (RR 1.57; 95% CI, 1.02, 2.43; p = 0.04, Fig. 4). No significant differences were observed between the groups in terms of aspiration/pneumonia rate (RR, 1.19; 95% CI, 0.51, 2.75; p = 0.69, Fig. 5A), diarrhea rate (RR, 0.82; 95% CI, 0.58, 1.16; p = 0.26, Fig. 5B), or increased GRV (RR, 1.05; 95% CI, 0.58, 1.90; p = 0.86, Fig. 5C). Moreover, no significant heterogeneity was detected in all analyses except for the aspiration/pneumonia rate, which was identified with moderate heterogeneity.
Sensitivity analysis and Baujat plot
In our leave-one-out sensitivity analysis (Table S2), a lower heterogeneity was reached after excluding MacLeod et al. [27] and Chen et al. [28] given the known moderate heterogeneity observed in aspiration/pneumonia rate. Despite this reduction in heterogeneity, consistent results were still concluded. On top of the “leave-one-out” sensitivity analysis, we conducted further exploration of heterogeneity using Baujat plot and identified Bonten et al. [31] as a potential outlier (Fig. S3). For other outcomes with low heterogeneity, the sensitivity analyses remained consistent results for the risk of increased gastric residual volume and diarrhea. However, there were divergent conclusions regarding the risk of mortality and constipation.
Publication bias
Funnel plot revealed no apparent asymmetry among the mortality rate from the included RCTs, indicating no publication bias (Fig. S2). However, because we included less than ten studies in the outcome analysis, distinguishing asymmetry was challenging.
Certainty of evidence
Based on the GRADE framework, the primary outcome was graded as having low certainty of evidence. Other secondary outcomes were graded as having very low certainty of evidence because of the small sample size and imprecision of the outcome measurement (Table S3).
Discussion
This meta-analysis involving 13 RCTs with 785 patients revealed that continuous feeding was associated with a lower mortality rate compared with intermittent feeding among critically ill patients in ICUs. In addition, continuous feeding exhibited similar risks of aspiration/pneumonia, increased GRV, and diarrhea as intermittent feeding. Therefore, our findings indicated that for critically ill patients, continuous feeding may be a more appropriate feeding modality than intermittent feeding. This finding is consistent with the recommendations of the ESPEN and ASPEN guidelines, which suggest that continuous feeding is more suitable for patients in ICUs because it reduces the diarrhea rate and increases feeding volume [2, 4]. Although our findings are consistent with clinical guidelines, the results from TSA suggest that false-positive may be present. Therefore, the observed reduction in mortality should be considered preliminary. According to the ASPEN guidelines, continuous feeding has shown the ability to achieve greater volume with fewer interruptions in delivery of EN, which may be reasonably account for the difference [4].
It is worth mentioning that we are the first article to observe the potential benefits of continuous feeding on the reduction of mortality rates. In contrast to our findings, previous meta-analyses [10, 12] have demonstrated that continuous feeding did not significantly reduce mortality. The nonsignificance may derive from methodological differences and flawed search strategies. Although the meta-analysis by Ma et al. enrolled 14 RCTs, only four RCTs with 339 patients were included in the pooled mortality rate [10], whereas this study analyzed seven RCTs with 548 patients. Another meta-analysis [12] conducted by Heffernan et al. not only failed to include the latest RCTs [20] but also included case-control studies [33]. Therefore, this study provides updated information and compelling evidence on the use of continuous feeding relative to the previous ones [10, 12]. However, the results of this meta-analysis should be interpreted with caution. The results of TSA neither support nor oppose the administration of continuous feeding. In other words, this study may have false-positive errors, and future large-scale rigorous randomized trials with other designs are warranted to provide more certainty regarding the clinical efficacy of various feeding modalities.
Regarding the secondary outcomes, our study found a significantly higher risk of constipation in patients receiving continuous feeding, which is consistent with the results of Heffernan et al. [12]. Nevertheless, the risk of constipation is affected by multiple factors, such as severity of disease, medication, and duration of bedridden status. The aforementioned confounding factors might influence the differences between the two feeding methods.
The pooled results for the aspiration/pneumonia rate exhibited moderate heterogeneity, which might be due to the various diagnostic approaches or criteria for aspiration or pneumonia. For example, the diagnosis of aspiration/pneumonia in most studies was based on the results of X-ray, blue-dye test, or clinical observation [20, 24, 26, 28,29,30, 32]. In addition, a previous study defined patients with “pneumonia” only when the criteria for ventilator-associated pneumonia were met [31], whereas another study did not describe the details of diagnosis [27]. To explore the heterogeneity, we conducted the Baujat plot and identified Bonten et al. [31] as the outlier, which might contribute the most to the presented overall heterogeneity (Fig. S3). In contrast to other included articles, Bonten et al. defined “aspiration pneumonia” only when the criteria for ventilator-associated pneumonia were met [31]. The stricter yet inconsistent diagnostic criteria might lead to the difference. Additionally, we performed a sensitivity analysis using the leave-one-out method. The results for the aspiration/pneumonia rate remain consistent during the leave-one-out process, while the heterogeneity was slightly and markedly reduced after excluding MacLeod et al. [27] and Chen et al. [28], respectively (Table S2).
High GRV is an acceptable surrogate of delayed gastric emptying, whereas the latter is a common finding among the critically ill or mechanically ventilated patients [34, 35]. Although our pooled results showed no significant differences in the GRV between the groups, various cutoffs for “increased GRV” in the included studies are a concern. Because higher GRV does not increase the risk for gastroesophageal reflux and associated aspiration [4, 36], a relatively conservative GRV cutoff may lead to a higher probability of unnecessary interruption of feeding, with no benefits in terms of reducing aspiration/pneumonia [37]. A clinical guideline has suggested holding EN for GRV < 500 ml in the absence of other signs of intolerance [4], and this can be used as a reference in future studies.
This study has several limitations. First, data for individualized nutritional requirements and feeding formula were lacking, which could be a factor influencing mortality rate. Second, several studies did not report the allocation concealment and blinding process, which might lead to selection, performance, and detection bias. Third, the small number of studies and insufficient sample sizes brought about the concern of imprecision. Finally, certain RCTs focusing on gastric acid pH, microbial colonization rates, or gastric cultures were excluded from our study due to limited clinical significance and misalignment with our primary research objectives, despite their inclusion in previous studies [33, 38, 39].
Conclusion
Compared with intermittent feeding, continuous feeding may reduce the mortality rate in critically ill patients. However, according to the GRADE framework, the certainty of evidence was regarded as low. TSA also indicated insufficient data to reach a firm conclusion. Further studies with larger sample sizes are warranted to provide more evidence for the use of continuous feeding in critically ill patients in ICUs and to elucidate the optimal feeding modality.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth. 2014;113:945–54.
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79.
Preiser JC, Arabi YM, Berger MM, Casaer M, McClave S, Montejo-González JC, et al. A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit Care. 2021;25:424.
McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2016;40:159–211.
Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early enteral nutrition provided within 24 h of ICU admission: a meta-analysis of randomized controlled trials. Crit Care Med. 2018;46:1049–56.
Ichimaru S. Methods of enteral nutrition administration in critically ill patients: continuous, cyclic, intermittent, and bolus feeding. Nutr Clin Pract. 2018;33:790–5.
Marik PE. Feeding critically ill patients the right 'whey': thinking outside of the box. A personal view. Ann Intensive Care. 2015;5:51.
Bear DE, Hart N, Puthucheary Z. Continuous or intermittent feeding: pros and cons. Curr Opin Crit Care. 2018;24:256–61.
Bolgeo T, Di Matteo R, Gallione C, Gatti D, Bertolotti M, Betti M, et al. Intragastric prepyloric enteral nutrition, bolus vs continuous in the adult patient: a systematic review and meta-analysis. Nutr Clin Pract. 2022;37:762–72.
Ma Y, Cheng J, Liu L, Chen K, Fang Y, Wang G, et al. Intermittent versus continuous enteral nutrition on feeding intolerance in critically ill adults: a meta-analysis of randomized controlled trials. Int J Nurs Stud. 2021;113:103783.
Thong D, Halim Z, Chia J, Chua F, Wong A. Systematic review and meta-analysis of the effectiveness of continuous vs intermittent enteral nutrition in critically ill adults. J Parenter Enteral Nutr. 2022;46:1243–57.
Heffernan AJ, Talekar C, Henain M, Purcell L, Palmer M, White H. Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis. Crit Care. 2022;26:325.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta‐analyses: application to a meta‐analysis of 65 trials. Stat Med. 2002;21:2641–52.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75.
Lee HY, Lee JK, Kim HJ, Ju DL, Lee SM, Lee J. Continuous versus intermittent enteral tube feeding forcritically ill patients: a prospective, randomized controlled trial. Nutrients. 2022;14:664.
McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158:183–94.
Nasiri M, Farsi Z, Ahangari M, Dadgari F. Comparison of intermittent and bolus enteral feeding methods on enteral feeding intolerance of patients with sepsis: a triple-blind controlled trial in intensive care units. Middle East J Dig Dis. 2017;9:218–27.
Evans DC, Forbes R, Jones C, Cotterman R, Njoku C, Thongrong C, et al. Continuous versus bolus tube feeds: does the modality affect glycemic variability, tube feeding volume, caloric intake, or insulin utilization? Int J Crit Illn Inj Sci. 2016;6:9–15.
Kadamani I, Itani M, Zahran E, Taha N. Incidence of aspiration and gastrointestinal complications in critically ill patients using continuous versus bolus infusion of enteral nutrition: a pseudo-randomised controlled trial. Aust Crit Care. 2014;27:188–93.
Tavares de Araujo VM, Gomes PC, Caporossi C. Enteral nutrition in critical patients; should the administration be continuous or intermittent? Nutr Hosp. 2014;29:563–7.
Maurya I, Pawar M, Garg R, Kaur M, Sood R. Comparison of respiratory quotient and resting energy expenditure in two regimens of enteral feeding—continuous vs. intermittent in head-injured critically ill patients. Saudi J Anaesth. 2011;5:195–201.
MacLeod JB, Lefton J, Houghton D, Roland C, Doherty J, Cohn SM, et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57–61.
Chen YC, Chou SS, Lin LH, Wu LF. The effect of intermittent nasogastric feeding on preventing aspiration pneumonia in ventilated critically ill patients. J Nurs Res. 2006;14:167–80.
Serpa LF, Kimura M, Faintuch J, Ceconello I. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:9–14.
Steevens EC, Lipscomb AF, Poole GV, Sacks GS. Comparison of continuous vs intermittent nasogastric enteral feeding in trauma patients: perceptions and practice. Nutr Clin Pract. 2002;17:118–22.
Bonten MJ, Gaillard CA, van der Hulst R, de Leeuw PW, van der Geest S, Stobberingh EE, et al. Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med. 1996;154:394–9.
Kocan MJ, Hickisch SM. A comparison of continuous and intermittent enteral nutrition in NICU patients. J Neurosci Nurs. 1986;18:333–7.
Spilker CA, Hinthorn DR, Pingleton SK. Intermittent enteral feeding in mechanically ventilated patients: the effect on gastric pH and gastric cultures. Chest. 1996;110:243–8.
Ladopoulos T, Giannaki M, Alexopoulou C, Proklou A, Pediaditis E, Kondili E. Gastrointestinal dysmotility in critically ill patients. Ann Gastroenterol. 2018;31:273–81.
Lew CC, Ong C, Mukhopadhyay A, Marshall A, Arabi YM. How to feed the critically ill—a review. Ann Acad Med Singapore. 2020;49:573–81.
Metheny NA, Schallom L, Oliver DA, Clouse RE. Gastric residual volume and aspiration in critically ill patients receiving gastric feedings. Am J Crit Care. 2008;17:512–9.
Poulard F, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. J Parenter Enteral Nutr. 2010;34:125–30.
Gowardman J, Sleigh J, Barnes N, Smith A, Havill J. Intermittent enteral nutrition—a comparative study examining the effect on gastric pH and microbial colonization rates. Anaesth Intensive Care. 2003;31:28–33.
Shahriari M, Rezaei E, Bakht LA, Abbasi S. Comparison of the effects of enteral feeding through the bolus and continuous methods on blood sugar and prealbumin levels in ICU inpatients. J Educ Health Promot. 2015;4:95.
Author information
Authors and Affiliations
Contributions
JYW, MYL, THL, CYK, KCH, YWT, WHH, MHC, PYH, CCL, and HTT contributed to this study, including the conception and design of the research. JYW, MYL, THL, CYK, KCH, and PYH performed data extraction, analyses, and interpretation of data, and CCL was a third reviewer to resolve any disagreements. JYW and THL assisted in statistics, and JYW and PYH ensured the certainty of evidence in accordance with the GRADE statement. JYW, MYL, THL, CYK, KCH, YWT, WHH, MHC, and PYH drafted the manuscript. This manuscript was revised by CCL and HTT. All authors gave final approval and agreed to all aspects of the work, ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, JY., Liu, MY., Liu, TH. et al. Clinical efficacy of enteral nutrition feeding modalities in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 77, 1026–1033 (2023). https://doi.org/10.1038/s41430-023-01313-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01313-8