Abstract
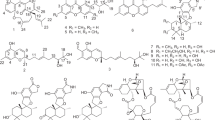
HPLC-UV-guided fractionation of crude extract from the marine sediment-derived bacterium Streptomyces sp. CNP-944 has yielded two new pyrazine alkaloids, actinopolymorphols E and F (1 and 2), in addition to the previously reported biosynthetic product, actinopolymorphol G (3), and the known actinopolymorphol D (4). The chemical structures of 1−3 were determined based on the interpretation of the 1D, 2D NMR, and MS spectroscopic data. Compound 2 displayed weak antibacterial activities against Kocuria rhizophila, Bacillus subtilis, and Staphylococcus aureus with minimum inhibitory concentration (MIC) values of 16, 64, and 64 μg ml−1, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sun W, Wu W, Liu X, Zaleta-Pinet DA, Clark BR. Bioactive compounds isolated from marine-derived microbes in China: 2009-18. Mar Drugs. 2019;17.6:339.
Knight V, Sanglier JJ, DiTullio D, Braccili S, Bonner P, Waters J, Zhang L. Diversifying microbial natural products for drug discovery. Appl Microbiol Biotechnol. 2003;62.5:446–58.
Khan ST, Komaki H, Motohashi K, Kozone I, Mukai A, Takagi M, Shin-ya K. Streptomyces associated with a marine sponge Haliclona sp.; biosynthetic genes for secondary metabolites and products. Environ Microbiol. 2011;13:391–403.
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2021;38:362–413.
Mincer TJ, Jensen PR, Kauffman CA, Fenical W. Appl Environ Microbiol. 2002;68:5005–11.
Anandan R, Dharumadurai D, Manogaran GP. An introduction to actinobacteria. Actinobacteria—Basic Biotechnol Appl. 2016;11:3–37.
Ōmura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. PNAS . 2001;98:12215–20.
Quinn GA, Banat AM, Abdelhameed AM, Banat IM. Streptomyces from traditional medicine: source of new innovations in antibiotic discovery. J Med Microbiol. 2020;69:1040–48.
Ayswaria R, Vasu V, Krishna R. Diverse endophytic Streptomyces species with dynamic metabolites and their meritorious applications: a critical review. Crit Rev Microbiol. 2020;46:750–8.
Lee L-H, Goh B-H, Chan K-G. Edoitorial: Antinobacteria: prolific producers of bioactive metabolites. Front Microbiol. 2020;11:1612.
Jose PA, Maharshi A, Jha B. Actinobacteria in natural products research: progress and prospects. Microbiol Res. 2021;246:126708.
Wu XA, Zhao YM, Yu NJ. A novel analgesic pyrazine derivative from the leaves of Croton tiglium L. J Asian Nat Prod Res. 2007;9.5:437–41.
Durán R, Zubía E, Ortega MJ, Naranjo S, Salvá J. Novel alkaloids from the red ascidian Botryllus leachi. Tetrahedron. 1999;55:13225–32.
Wyatt MA, Magarvey NA. Optimizing dimodular nonribosomal peptide synthetases and natural dipeptides in an Escherichia coli heterologous host. Biochem Cell Biol. 2013;91:203–8.
Wyatt MA, Mok MCY, Junop M, Magarvey NA. Heterologous expression and structural characterisation of a pyrazinone natural product assembly line. Chem Bio Chem. 2012;13:2048–15.
Romero CA, Grkovic T, Han J, Zhang L, French JRJ, Kurtbӧke DI, Quinn RJ. NMR fingerprints, an integrated approach to uncover the unique components of the drug-like natural product metabolome of termite gut- associated Streptomyces species. RSC Adv. 2015;5:104524–34.
Ohta A, Okuwaki Y, Komaru T, Hisatome M, Yoshida Y, Aizawa J, Nakano Y, Shibata H, Miyazaki T, Watanabe T. Catalytic hydrogenation of 2,5-dialkylpyrazines and 3,6-dialkyl-2-hydroxypyarazines. Heterocycles. 1987;26:2691–701.
Rojas N, Grillasca Y, Acosta A, Audelo I, Mora GG. A new method for the synthesis of symmetrical disubstituted pyrazines. J Heterocycl Chem. 2013;50:982–4.
Ohta A, Akita Y, Nakane Y. Conversion of 2,5-diphenyl- and 2,5-dibenzyl-pyrazines to 2,5-diketopiperazines. Chem Pharm Bull. 1979;27:2980–7.
Daw P, Kumar A, Espinoas-Jalapa NA, Diskin-Posner Y, Ben-David Y, Milstein D. ACS Catal. 2018;8:7737–41.
Murray KE, Shipton J, Whitfield FB. 2-Methoxypyrazines and the flavour of green peas (Pisum sativum). Chem Ind. 1970;4:897–8.
Chen T-B, Reineccius GA, Bjorklund JA, Leete E. Biosynthesis of 2-methoxy-3-isopropylpyrazine in Pseudomonas perolens. J Agric Food Chem. 1991;39:1009–12.
MacDonald JC. Biosynthesis of pulcherriminic acid. Biochem J. 1965;96:533–8.
MacDonald JC. Biosynthesis of hydroxyaspergillic acid. J Biol Chem. 1962;237:1977–81.
Maha A, Rukachaisirikul V, Saithong S, Phongpaichit S, Poonsuwan W, Sakayaroj J, Hannongbua S. Terezine derivatives from the fungus Phoma herbarum PSU-H256. Phytochemistry. 2016;122:223–9.
Mortzfeld FB, Hashem C, Vranková K, Winkler M, Rudroff F. Pyrazines: synthesis and industrial application of these valuable flavor and fragrance compounds. Biotechnol J. 2020;15:2000064.
Shimoda M, Nakada Y, Nakashima M, Osajima Y. Quantitative comparison of volatile flavor compounds in deep-roasted and light-roasted sesame seed oil. J Agric Food Chem. 1997;45:3193–6.
Opletalová V, Hartl J, Patel A, Palat K Jr, Buchta V. Ring substituted 3-phenyl-1-(2-pyrazinyl)−2-propen-1-ones as potential photosynthesis-inhibiting, antifungal and antimycobacterial agents. ΙL Farm. 2002;57:135–44.
Huang S-X, Powell E, Rajski SR, Zhao L-X, Jiang C-L, Duan Y, Xu W, Shen B. Discovery and total synthesis of a new estrogen receptor heterodimerizing actinopolymorphol A from Actinoppolymorpha rutilus. Org Lett. 2010;12:3525–7.
Acknowledgements
This research was supported by the National Research Foundation of Korea grant funded by the Korean Government (Ministry of Science and ICT; no. 2021R1A4A2001251 to S-JN) and in part by the project titled “Development of Potential Antibiotic Compounds Using Polar Organism Resources” (15250103, KOPRI grant PM21030 to S-SC) funded by the Ministry of Oceans and Fisheries, Korea. Isolation of the bacterium strain Streptomyces sp. CNP-944 was a result of financial support from the US National Cancer Institute (grant CA R37044848 to WF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Hillman, P.F., Lee, J.Y. et al. Actinopolymorphols E and F, pyrazine alkaloids from a marine sediment-derived bacterium Streptomyces sp. J Antibiot 75, 619–625 (2022). https://doi.org/10.1038/s41429-022-00562-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00562-2