Abstract
Two cyclotetrapeptides, henceforth named Provipeptides A (1) and B (2), along with five known diketopiperazines (3–7) were isolated from the liquid culture of marine Streptomyces sp. 161a recovered from a sample of sea grass Bryopsis sp. The structures of cyclotetrapeptides and diketopiperazines (DKPs) were established by 1D and 2D NMR data, MS, and by comparison with literature data. The absolute stereochemistry of compounds cyclo-(l-Pro-l-Leu-d-Pro-l-Phe) 1 and cyclo-(-Pro-Ile-Pro-Phe) 2 was established by the Marfey’s method. Compound 1 showed antibacterial activity against rice phytopathogenic strains Burkholderia glumae (MIC = 1.1 mM) and Burkholderia gladioli (MIC = 0.068 mM), compound 2 was active only against B. glumae (MIC = 1.1 mM), and DKP cyclo-[l-Pro-l-Leu] 5 showed to be active against B. gladioli (MIC = 0.3 mM) and B. glumae (MIC = 2.4 mM). Compounds 1 and 2 showed 65% and 50% inhibition of Colletotrichum gloeosporioides (yam pathogen) conidia germination, respectively at a concentration of 1.1 mM.
Similar content being viewed by others
Introduction
In Colombia, rice is the third most important crop in the country [1] and has been affected by several bacterial phytopathogens including Burkholderia spp., causing bacterial panicle blight disease. On the other hand, Colombia produces more than 385,000 tons per year of yam (Dioscorea spp.), which is a monocotyledon plant characterized by having fleshy tubers called yams containing abundant starch.
This species is widely distributed in regions with high tropical and subtropical rainfall rates and is used mainly for human nutrition in places such as Africa, South Asia, the Pacific Islands and the Colombian Caribbean region. It is a basic product in the diet of the population for its high nutritional content, being starch its main component a rich source of carbohydrates; also for its high content of vitamin C, with its antiscurvy properties, making it a valuable food for humans and animals [2]. This crop is affected by anthracnose, which is caused by the fungal phytopathogen Colletotrichum gloeosporioides, generating production losses of up to 75% [2]. In view of that, a search for efficient strategies for the control of these phytophatogen is required. During the second half of the twentieth century, one of the major concerns in agriculture was focused on the pollution originated by the extensive use of highly toxic agrochemicals such as pesticides [3, 4]. Studies since the 1970s have shown that, besides the harmful effects at the public health level, the use of pesticides has led to phytopathogen resistance caused by the systematic use of agrochemicals [5], evincing the importance of seeking environmentally friendly compounds for the control of phytopathogens.
In recent years, marine microorganisms have gained great relevance as a source of secondary metabolites with potential to be used for the control of phytopathogens [6,7,8,9]. Marine-derived metabolites become prototypes for the development of new substances with a putative insecticidal and antimicrobial potential, making them excellent candidates for use in the agricultural sector [10]. Actinobacteria are ubiquitous in the marine environment and one of the most prolific source of natural products among marine microorganisms and continue to surprise with the versatility of their biosynthetic machinery. The structural novelty and diversity of actinobacteria secondary metabolites still make them a prolific source of new leads for antibiotics discovery and development [6, 11, 12]. In addition, about two-thirds of all biologically active compounds produced by actinobacteria are produced by bacteria belonging to the genus Streptomyces [13].
In this paper, as a part of our search for bioactive compounds for the control of phytopathogens [14], we present the isolation of antimicrobial compounds from the strain Streptomyces PNM-161a isolated from a sample of Bryopsis sp. (Chlorophyta) as a source of active compounds against Burkholderia glumae, Burkholderia gladioli (rice pathogens), and a strain of C. gloeosporioides (yam pathogen) [14]. Overall, we describe isolation, structural elucidation and biological activities of two cyclic tetrapeptides along with 5 DKPs from Streptomyces sp. PNM-161a.
Materials and methods
General
1H and 13C NMR (1D and 2D) spectra were recorded on a Bruker Advance 400 spectrometer (400 MHz for 1H and 100 MHz for 13C) using CDCl3 (7.26 ppm and 77.0 ppm), CD3OD (3.35 ppm and 49.0 ppm) and DMSO-d6 (2.50 ppm and 39.5 ppm) as solvents, and residual solvent signals were used as internal standards. High-resolution mass data were collected on an accurate-mass quadrupole Time-of-Flight (q-TOF) (Agilent Technologies) mass spectrometer, ESI positive mode. Nebulizer 50 (psi); Gas Flow 10 l min−1; Gas Temp 350 °C. Fragmentor 175V, Skimmer 75V, Vpp 750V.
Column chromatography was carried out under vacuum using silica gel 60G Merck (0.063–0.200 mm), and flash chromatography was carried out on silica gel 60 (70-230, Macherey-Nagel®). HPLC-ELSD was performed on a Thermo Dionex ultimate 3000 system coupled to an ELSD Sedex 85 (Sedere, France) detector (Gain detector 10 and temperature 80 °C). Optical rotations were measured on a Polartronic E, Schmidt+Haensch polarimeter in 1 ml × 5 cm cells. For reading the antibacterial bioassay in 96-well plates, an AccuReader Metertech (Taipei, Taiwan) was used reading at 600 nm. All solvents used were HPLC (High Performance Liquid Chromatography) grade.
ISP-2 medium contained 4 g of malt extract, 10 g of dextrose, and 4 g of agar per liter of tap water. Luria-Bertani (LB) medium contained, 5 g yeast extract, 10 g NaCl, 10 g tryptone, per liter of tap water, adjusting pH at 7.2 using NaOH 3 M. King’s B medium (KB) contained 10 g peptone, 1.5 g anhydrous KH2PO4, 15 g glycerol, and 1.5 g MgSO4 per liter of tap water.
Fermentation and extraction
Streptomyces sp. PNM-161a was originally isolated from a sample of Bryopsis sp. (Chlorophyta) [14]. DNA 16S rRNA nucleotide sequence for this strain was deposited in GenBank/EMBL/DDBJ, under accession number KX641396 [14]. For the present study, the isolate was recovered from its cryopreserved stock and cultured into ISP-2 plates at 28 °C for one week. A two-step culture was performed for extract production, consisting of an initial inoculum of Streptomyces sp. PNM-161a into a primary culture of 100 ml in LB medium in 500 ml conical flasks by shaking at 130 rpm at 25 °C for 5 days. This 100 ml preculture was transferred to a 5 l flask containing 900 ml of LB medium to complete 1 l of culture per flask (40 flasks for a total of 40 l culture). The flask was shaken at 130 rpm and 25 °C for 10 days. The biomass of each flask was removed by centrifugation at 5000 rpm for 15 min. The culture supernatant was extracted three times with ethyl acetate (1:1). The organic layers of the 40 culture flasks were pooled and then evaporated under vacuum to obtain 3.0 g of crude extract.
Isolation of compounds
The crude extract was fractionated using a solid phase extraction (SPE) cartridge (Phenomenex Strata C-18, 5 g, 20 ml) with a MeOH/H2O discontinuous gradient solvent of 10, 30, 50, 70 and 100% MeOH (30 ml each) to obtain five fractions F1–F5. Fraction F4 (860 mg) obtained from the 70% MeOH showed antimicrobial activity (vide infra) and was chromatographed on silica gel 60 using a discontinuous gradient solvent from CHCl3 100% to MeOH 100% to obtain 170 fractions of 10 ml pooled in 11 fractions F4.1–F4.11 by their TLC profiles. Fraction F4.1 (100% CHCl3) yielded compound 1 (138 mg). Fraction F4.2 (430 mg, eluted with CHCl3/MeOH 98:2) was subjected to HPLC using a Kromasil C8 column (10 × 250 mm and 10 µm) with an isocratic solvent system of MeCN 15% and water 85% (2 ml min−1) to yield compound 1 (280 mg), compound 2 (29,2 mg) and compound 5 (5 mg). Fraction F4.3 (51 mg, eluted with CHCl3/MeOH 96:4) was subjected to HPLC using a Kromasil C8 column (10 × 250 mm and 10 µm) and eluted with a gradient of MeCN (A) and water (B), as follows: 10% A with a linear increase of up to 25% A in 20 min, followed by 10 min at 25% A; then a linear increase of up to 30% A in 10 min, followed by a linear increase of up to 50% A in 10 min, and a final linear increase of up to 100% A in 3 min followed by 3 min at 100% A to yield compound 3 (5 mg), compound 4 (1 mg), and compound 6 (4 mg). Fraction F4.4 (59 mg, eluted in CHCl3/MeOH 94:6) was fractionated by HPLC. The chromatographic analysis used the same chromatographic column mentioned above at a flow rate of 2.0 ml min−1. The mobile phase consisted of a gradient of MeCN (A) and water (B), as follows: a linear increase from 5% to up to 25% A in 30 min, followed by a linear increase of up to 70% A in 3 min and then by 5 min at 70% A. This fractionation yielded compound 7 (10 mg).
Marfey analysis
The absolute configurations of the amino acids present in 1 and 2 were determined by the Marfey’s method [15, 16]. Briefly, solutions at 50 μM of each l-amino acid (proline, leucine, isoleucine and phenylalanine) were prepared. Then, 50 μl of each solution was treated with a 1-fluoro-2,4-dinitrophenyl-5-l-alaninamide (l-FDAA) at 1% in acetone (100 μl) and sodium bicarbonate 1 M (20 μl). The mixture was heated for 1 h at 50 °C. Subsequently, the mixture was cooled to room temperature, and a solution of HCl 1 M (10 μl) was added. The obtained residue was dried in a desiccator over NaOH pellets and dissolved in DMSO (1 ml). The same procedure was repeated employing 1-fluoro-2,4-dinitrophenyl-5-d-alaninamide (d-FDAA). HPLC analyses were performed in a HPLC-DAD (agilent 1260), symmetry C-18 column (100 × 4.6 mm, 3.5 μm) eluting with a first step gradient (MeCN–HCOOH 0,1%) from 10:90 up to 18:82 in 30 min, followed by a second gradient from 18:82 up to 40:60 in 15 min, and maintaining 40:40 for other 10 min for a total run time of 55 min, at a flow rate of 2 ml min−1 and 25 °C, and detecting on a DAD detector.
For the hydrolysis of 1 and 2, the compounds (1 mg each) were treated with a 1.5 M HCl solution (1 ml) and heated for 24 h at 110 °C. The mixture was dried in a desiccator over NaOH pellets and the residue was dissolved in water. The obtained solution of each compound (100 μl) was derivatized with l- or d-FDAA reagent 1% in acetone. The mixture was heated at 50 °C for 24 h, and then cooled to room temperature. Then, HCl 2 M (20 μl) was added to the mixture. The obtained solution was dissolved in formic acid 0.01 M/MeCN (1:3) to complete a final volume of 1 ml. This sample was analyzed by HPLC employing the conditions described above. Retention times and UV spectra of each chromatographic peak were compared with those obtained for the amino acid derivatives previously obtained.
Antimicrobial activity of fraction and pure compounds
Strains of Burkholderia spp and C. gloeosporioides 26B were kindly provided by the Biotechnology Institute of the Universidad Nacional de Colombia. The antibacterial activity analysis of the compounds isolated from Streptomyces sp. PNM-161a, against bacterial phytopathogens B. glumae (ATCC 33617) and B. gladioli (3704-1-FEDEARROZ), was performed in 96-well plates using the broth dilution method [16]. Briefly, 190 µl of KB medium and 10 µl of each bacterial phytopathogen (Absorbance λ600 nm = 0.25) were cultured in each well. The amount of 200 µl of a solution of 1000 µg ml−1 of pure compounds and positive control (oxolinic acid) previously dissolved in MeOH 10% was applied in the first vertical line of the plate. Serial dilutions were made to obtain five different concentrations in each well: 500, 250, 125, 62.5, 32.25, 16.12 and 8.1 µg ml−1. Twenty microliters of the ethyl acetate extract previously dissolved in MeOH 10% of the LB media culture was used as negative controls, respectively. The plates were incubated at 37 °C for 24 h, growth inhibition was measured by absorbance at λ = 600 nm [16].
For the antifungal testing, the phytopathogenic fungus C. gloeosporioides 26B obtained from yam plants with classical symptoms of anthracnose [17] was tested. Phytopathogenic fungi were kindly provided by the Instituto de Biotecnología—Universidad Nacional de Colombia. The antifungal activities of compounds 1, 2 and 3 were determined using liquid cultures in 96-well plates using a modification of the broth microdilution method [16]. The medium volume per well was 100 μl. These fungi were inoculated in RPMI-1640 medium. As for the antifungal activity evaluation of pure compounds, the final concentrations of pure compounds in the wells were 500, 250, 125, 62.5 µg ml−1 and 32.25, 16.12, and 8.1 µg ml−1. All tested compounds were previously dissolved in MeOH 10% and applied in the first vertical line of the plate. The microorganism suspensions (10 μl) of each pathogenic fungus were added to a 96-well microtiter plate. The culture media (20 µl of MeOH 10%) and a solution of 1% clotrimazole (5 μl well−1) were used as negative and positive control, respectively. The plates were incubated at 25 °C for 48 h, growth inhibition was measured by absorbance at λ = 600 nm.
The minimum inhibitory concentration (MIC) for both, the antibacterial and the antifungal tests, was determined as the lowest concentration that inhibited visible growth of phytopathogens. The growth inhibition of each dilution was calculated using the following formula: % inhibition = 100 × [1 − OD of treated well/OD of negative control well]. The dilutions of the tested compounds were independently performed at least three times.
Results and discussion
Isolation and purification of bioactive compounds
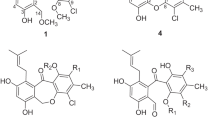
In our previous work, the ethyl acetate extract from Streptomyces sp. PNM-161a showed broad spectra of antimicrobial activity against rice and yam phytopathogens [14]. The phylogenetic analysis showed that its closest neighbors were Streptomyces griseochromogenes, Streptomyces violascens, Streptomyces resistomycificus, Streptomyces exfoliates, Streptomyces albidoflavus, all of them with a 99.86% of similarity [14], making these species a prolific source of bioactive compounds. To identify the production of bioactive compounds, Streptomyces sp. PNM-161a was cultured in LB medium (40 l), extracted in ethyl acetate and fractionated by SPE (see methodology). Fraction F4 (70% MeOH), selected because of its potent antibacterial activity against B. glumae and B. gladioli (Table 1S, Fig. 1S, see supplementary information), allowed isolating seven compounds (Fig. 1).
Compound 1 was isolated as a solid with \([\alpha]_{\rm{D}}^{23}\) -15.04 (c 0.1, MeOH) and showed a sodium adduct ion at m/z 477.2478 by HRESIMS (High-resolution Electrospray ionisation mass spectrometry), consistent with the molecular formula C25H34N4O4 (calcd for C25H34N4O4 Na m/z = 477.2483, Δ 1.04 ppm, 0.49 mmu). 1H NMR (400 MHz, CD3OD, Table 1) showed characteristic signals for a peptide with a single major conformation, with signals for an NH amide proton at δH 7.91 (1H, bs), a monosubstituted aromatic ring at δH 7.31–7.22 (5H, m), four α methine protons at δH 4.45 (1H, dt, J = 4.9, 1.6 Hz), 4.25 (1H, dd, J = 7.7, 1.5 Hz), 4.13 (1H, m) and 4.07 (1H, dd, J = 6.5, 1.7 Hz), suggesting that 1 was comprised of four amino acid residues. In addition, eight methylenes at δH 3.54 (1H, m)−δH 3.38 (1H, m); δH 3.52 (1H, m) −3.35 (1H, m); δH 3.17 (2H, dd, J = 4.9, 2.5 Hz); δH 2.30 (1H, m)−δH 1.94 (1H, m); δH 2.11 (1H, m)−δH 1.27 (1H, m); δH 2.02 (2H, m), 1.92 (1H, m) −1.52 (1H, t, J = 7.7 Hz), δH 1.80 (2H, m) were observed, along with one methyne at δH 1.89 (1 H, m), and two methyles at δH 0.97 (3H, d, J = 6.3 Hz) and δH 0.95 (3H, d, J = 6.3 Hz). Spectra 13C NMR and HSQC (Heteronuclear single quantum correlation experiment) (100 MHz, CD3OD, Table 1) showed the presence of four amide carbonyl resonances at δC 172.8, 170.9, 168.9 and 166.9, along with the four α-CH groups at δC 60.3, 60.1, 57.8, and 54.6, confirming the presence of four amino acids. In addition, the spectra showed one aromatic ring with signals at δC 137.3, 131.0 (×2), 129.5 (×2) and 128.1; eight methylenes at δC 46.5, 45.9, 39.4, 38.7, 29.4, 29.3, 23.6, 23.3, one methine at δC 25.7 and two aliphatic methyl carbons at δC 22.7 and δC 22.2.
2D NMR spectroscopic data from compound 1 (COSY (Correlated spectroscopy), HMBC (Heteronuclear multiple bond correlation experiment), and HSQC) were consistent with a tetrapeptide composed of one phenylalanine, one leucine and two proline residues. The amino acid sequence was determined from the observed key HMBC correlations (CD3OD), as follows (Fig. 2 and Table 1): the δ proton of Pro-1 (δH 3.52) correlated to the Leu carbonyl group (δC 168.9); the α (δH 4.07) and δ (δH 3.38) protons of Pro-2 correlated to the Phe carbonyl group at δC 166.9. A further HMBC experiment measured in DMSO-d6, (Fig. 11S, supplementary information) showed correlations between the proton signal, assigned to N–H Phe (δH 7.98) with the carbonyl carbon of Phe (δC 165.1) and with the carbonyl carbon of Pro-1 (δC 170.4); and α Pro-1 (δC 58.5) (Fig. 2). A cyclic structure for compound 1 is proposed by correlations of N–H from Leu (δH 8.01) to α Pro-1 (δC 58.4) and to the carbonyl of Pro-2 (δC 169.0), thus confirming the structure of compound 1 as presented in Fig. 1.
The absolute configuration of the amino acids from compound 1 was established by the Marfey’s method. The mixture obtained after hydrolyzing compound 1 and further derivatization with l-FDAA was analyzed by HPLC-DAD. The chromatogram showed three peaks with retention times of 29.9, 33.9, 41.57 and 42.26 min, which were consistent with the retention times and the UV spectra obtained for l-Pro, d-Pro, l-Leu, and l-Phe standards and different from those obtained for d-FDAA (Fig. 12S, see supplementary information). The entire analysis allowed determining that compound 1 is a cyclic tetrapeptide conformed by residues of l-Phe, l-Leu, and both l- and d-Pro, and its structure is depicted in Fig. 2. This structure is an stereoisomer of the the cyclic tetrapeptide cyclo-(l-prolyl-l-leucyl-l-prolyl- l-phenylalanyl) produced by a marine strain of Pseudomonas sp. isolated from the sponge Halisarca ectofibrosa collected in Okinawa, Japan [18]. The peptide sequence of 1 was established as cyclo-(l-prolyl-l-leucyl-d-prolyl-l-phenylalanyl) by HMBC (Fig. 2). A similar compound was isolated by Rungprom [18] and synthetized by Dahiya [19], and the NMR data published for the natural and the synthetic compound cyclo-(l-prolyl-l-leucyl-l-prolyl- l-phenylalanyl) are the same. However the NMR data of compound 1 are different from those published by Rungprom [18] and Dahiya [19], suggesting that 1 corresponds to an stereoisomer of the published compound. The main difference was the proton chemical shift of α Pro-2 at δH 2.62 for the synthetic compound and δH 4.07 for compound 1. This fact allowed us to propose d-Pro in position 2, and thus proposing the structure of 1 as is presented in Fig. 1. This proposal was confirmed by computational models of compound 1 and the stereoisomer synthetized by Dahiya [19], obtained by Free Maestro ver 11.4 software using Universal Force Field (UFF), where the model obtained for compound 1 do not present anisotropic effect from the Phe aromatic ring to the α Pro-2 proton, while the compound cyclo-(l-prolyl-l-leucyl-l-prolyl- l-phenylalanyl) does present such anisotropic effect as can be seen in Fig. 13S (see supplementary information).
Compound 2 was isolated as a solid with \([\alpha]_{{\rm{D}}}^{23}\) + 0.31 (c 0.1, MeOH) and showed a molecular ion peak at m/z 455.2649 for the pseudomolecular ion [M + H]+ by HRESIMS, consistent with the molecular formula C25H34N4O4 (calcd for C25H35N4O4 m/z = 455.2663, Δ 3.07 ppm, 1.4 mmu). 1H NMR (400 MHz, CD3OD, Table 1) showed that compound 2 was a tetrapeptide similar to 1, but bearing an isoleucine residue with signals at δH 4.07 (1H, dd, J = 9.6, 1.9 Hz), δH 2.17 (1H, dqdd, 9.6, 7.0, 4.5, 2.4 Hz), δH 1.45 (1H, dqd 13.4, 7.5, 4.5 Hz)−δH 1.33 (1H, dqd, 13.4, 7.5, 2.4 Hz), 1.07 (3H, d, J = 7.0 Hz) δH 0.93 (3H, t, J = 7.5 Hz), instead of the leucine residue. 13C NMR (100 MHz, CD3OD, Table 1) confirmed the presence of the Ile residue with signals at 167.5 (C), 61.3 (CH), 37.1 (CH), 25.4 (CH2), 12.5(CH3), 15.5 (CH3).
The amino acid sequence was determined from the observed key HMBC correlations shown in Fig. 1, where the Ile unit was placed between the two Pro units by the correlations from the δ-proton of Pro-1 (δH 3.50) to the Ile carbonyl (δC 167.5) and from the α Ile proton (δH 4.07) to the Pro-2 carbonyl (δC 170.9). The Phe was bonded to Pro-2 as established by the correlation from Pro-2 α-proton (δH 4.09) and δ-proton (δH 3.37) to the Phe carbonyl (δC 166.9). The HMBC experiment in DMSO-d6 (Fig. 14S, supplementary information) showed the correlations between the Phe NH proton (δH 7.91) to its own carbonyl (δC 166.9) and to the α-carbon of Pro-1 (δC 60.1), as displayed in Fig. 2, allowing identifying compound 2 as cyclo-(Pro-Ile-Pro-Phe), and its planar structure is presented in Fig. 1. To the best of our knowledge, compound 2 has not been previously reported in the literature.
In addition, in the present study, five diketopiperazines were isolated and identified as cyclo-(l-Phe-d-Pro) 3 [19], cyclo-(l-Phe-l-Pro) 4 [20], cyclo-(l-Pro-l-Leu) 5 [21], cyclo-(l-Met-l-Pro) 6 [22], cyclo-(l-Tyr-l-Pro) 7 [23, 24], on the basis of their NMR and MS data and by comparison with those reported in the literature; the structures are shown in Fig. 1.
Antimicrobial activity evaluation
The evaluation of MIC for compounds 1 to 7 against rice phytopathogen bacterial strains of B. glumae and B. gladioli is shown in Table 2. Compounds 1 and 2 exhibited the highest antibacterial activity against both bacteria with values of MIC = 1.1 mM against B. glumae. Interestingly, compound 1 showed to be active against B. gladioli (MIC = 0.068 mM) with a very similar value to that of oxolinic acid, whereas compound 2 was not active against the same bacteria. In addition, compound 5 exerted selectivity to inhibit B. gladioli (MIC = 0.29 mM). The remaining compounds showed mild activities with values between 1.8 and 2.19 mM, and compound 7 was not active at the conditions evaluated. Furthermore, compounds 1 and 2 were tested against C. gloeosporioides at a concentration of 1.1 mM, showing 65% and 50% germination inhibition of the C. gloeosporioides conidia, respectively. This test allowed identifying tetrapeptides as the compounds responsible of the bioactivity observed in the crude extract of Streptomyces sp. PNM-161a.
In summary, two new cyclic tetrapeptides 1 and 2, along with 5 known diketopiperazines 3–7, were isolated from the strain Streptomyces sp. PNM-161a cultured in LB broth. The bioactivity assays evinced that compound 1 (B. gladioli MIC = 0.068 mM; B. glumae MIC = 1.1 mM), compound 2 (B. glumae MIC = 1.1 mM) and the diketopiperazine 5 (B. gladioli MIC = 0.3 mM; B. glumae MIC = 2.4 mM) were the compounds responsible for the antimicrobial activity against bacterial and fungal phytopathogens here tested.
References
Chica J, Yeimy C, Tirado O, Barreto JM. Indicadores de competitividad del cultivo del arroz en Colombia y Estados Unidos. Rev Cienc Agrícolas. 2016;33:16. http://revistas.udenar.edu.co/index.php/rfacia/article/view/3097.
Giraldo Marroquín N, Bustamante Rodríguez SL, Pinzón Gutiérrez YA, Buitrago Hurtado G. Caracterización molecular de Colletotrichum gloeosporioides aislado de plantas de ñame de la Costa Atlántica Colombiana utilizando la técnica “DNA Amplification Fingerprinting (DAF). Rev Colomb Biotecnol. 2016;18:95–103.
Rai MK, Kalia RK, Singh R, Gangola MP, Dhawan AK. Developing stress tolerant plants through in vitro selection—an overview of the recent progress. Environ Exp Bot. 2011;71:89–98.
Prévost K, Couture G, Shipley B, Brzezinski R, Beaulieu C. Effect of chitosan and a biocontrol streptomycete on field and potato tuber bacterial communities. BioControl. 2006;51:533–46.
Aktar W, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2009;2:1–12.
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep [Internet]. 2017;34:235–94. http://xlink.rsc.org/?DOI=C6NP00124F.
Kong, Q. Marine microorganisms as biocontrol agents against fungal phytopathogens and mycotoxins. Biocontrol Science and Technology. 2018;28:77–93. https://doi.org/10.1080/09583157.2017.1419164.
Manivasagan P, Venkatesan J, Sivakumar K, Kim SK. Marine actinobacterial metabolites: current status and future perspectives. Microbiol Res. 2013;168:311–32. https://doi.org/10.1016/j.micres.2013.02.002.
Newman DJ. Developing natural product drugs: Supply problems and how they have been overcome. Pharmacol Ther. 2016;162:1–9. https://doi.org/10.1016/j.pharmthera.2015.12.002.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35.
Hassan SSul, Shaikh AL. Marine actinobacteria as a drug treasure house. Biomed Pharmacother. 2017;87:46–57.
ul Hassan SS, Anjum K, Abbas SQ, Akhter N, Shagufta BI, SAA Shah, et al. Emerging biopharmaceuticals from marine actinobacteria. Environ Toxicol Pharmacol. 2017;49:34–47.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77.
Betancur LA, Naranjo-Gaybor SJ, Vinchira-Villarraga DM, Moreno-Sarmiento NC, Maldonado LA, Suarez-Moreno ZR, et al. Marine actinobacteria as a source of compounds for phytopathogen control: an integrative metabolic-profiling/bioactivity and taxonomical approach. Virolle M-J, editor. PLoS ONE. 2017;12:e0170148. http://dx.plos.org/10.1371/journal.pone.0170148.
Marfey P Determination of o-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene by [Internet]. Vol. 49, Carlsberg Res. Commun. 1984. https://link.springer.com/content/pdf/10.1007%2FBF02908688.pdf.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71–9. http://linkinghub.elsevier.com/retrieve/pii/S2095177915300150.
Moreno-Moran M, Burbano-Figueroa O. First report of Colletotrichum gossypii var. cephalosporioides causing cotton ramulosis in Colombia. Plant Dis. 2016 Mar;100:653–653.
Rungprom W, Siwu ERO, Lambert LK, Dechsakulwatana C, Barden MC, Kokpol U, et al. Cyclic tetrapeptides from marine bacteria associated with the seaweed Diginea sp. and the sponge Halisarca ectofibrosa. Tetrahedron. 2008;64:3147–52.
Rajiv D, Hemendra G. Synthesis and pharmacological studies on a cyclooligopeptide from marine bacteria. Chin J Chem. 2011;29:1911–6.
Li X, Dobretsov S, Xu Y, Xiao X, Hung O, Qian PY. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling. 2006;22:201–8.
Bao J, Zhang XY, Xu XY, He F, Nong XH, Qi SH. New cyclic tetrapeptides and asteltoxins from gorgonian-derived fungus Aspergillus sp. SCSGAF 0076. Tetrahedron. 2013;69:2113–7.
Kalinovskaya NI, Romanenko LA, Kalinovsky AI. Antibacterial low-molecular-weight compounds produced by the marine bacterium Rheinheimera japonica KMM 9513T. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol. 2017;110:719–26.
Kumar N, Mohandas C, Nambisan B, Kumar DRS, Lankalapalli RS. Isolation of proline-based cyclic dipeptides from Bacillus sp. N strain associated with rhabitid entomopathogenic nematode and its antimicrobial properties. World J Microbiol Biotechnol. 2013;29:355–64.
Wattana-Amorn P, Charoenwongsa W, Williams C, Crump MP, Apichaisataienchote B. Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22-4 against phytopathogenic bacteria. Nat Prod Res. 2016;30:1980–3.
Acknowledgements
This study was conducted with the financial support of COLCIENCIAS (grant Cod. 1101-659-44402 CT.537/14) and International Foundation for Science IFS, Uppsala-Sweden (grant Cod. 5023–2). Universidad Nacional de Colombia DIEB grant 37151. L.A.B thanks the Programa Doctoral Becas Colciencias (567) for the granted scholarship (2012), Universidad de Caldas for the leave of absence for doctoral studies. The ANLA (Agencia Nacional de Licencias Ambientales) and the Ministerio de Ambiente y Desarrollo Sostenible granted permission to collect samples and perform this research (Permission No 4 of 10/02/2010, Anexo 2, Contrato de Acceso a Recurso Genético No 108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Betancur, L.A., Forero, A.M., Romero-Otero, A. et al. Cyclic tetrapeptides from the marine strain Streptomyces sp. PNM-161a with activity against rice and yam phytopathogens. J Antibiot 72, 744–751 (2019). https://doi.org/10.1038/s41429-019-0201-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0201-0