Abstract
This manuscript describes a single pot protocol for the selective introduction of unprotected sugars to the C3 position of the cardiotonic steroid strophanthidol. These reactions proceed with high levels of regiocontrol (>20:1 rr) in the presence of three other hydroxyl functionalities including the C19 primary hydroxyl group and could be applied to different sugars to provide the deprotected cardiac glycosides upon work up (5 examples, 77–69% yield per single operation). The selective glycosylation of the less reactive C3 position is accomplished by the use of traceless protection with methylboronic acid that blocks the C5 and C19 hydroxyls by forming a cyclic boronic ester, followed by in situ glycosylation and a work up with ammonia in methanol to remove the boronic ester and the carbohydrate ester protecting groups.
Similar content being viewed by others
Introduction
Cardiotonic steroids represent a large family of natural products with a diverse range of biological activities [1,2,3,4,5,6,7]. These molecules have been considered of great importance for human and animal health due to their involvement in the regulation of such crucial functions as renal sodium transport and arterial pressure, as well their roles in modulation of cell growth, differentiation, apoptosis, fibrosis, immunity and carbohydrate metabolism, and the control of various central nervous functions and behavior [8,9,10,11,12,13]. Due to their wide spectrum of biological activities including cardiotonic, anticancer [14], anti-viral [15], immunoregulatory [16], neural outgrowth differentiative [17], anti-inflammatory [18], and anti-hypertensive [19], cardiotonic steroids have been evaluated as potential therapeutic agents in a variety of recent studies. An overwhelming amount of data (including several clinical trials) indicates that cardiotonic steroids hold great potential as the therapeutics for treating cancer [20]. It is suggested that the steroid interaction with Na+/K+-ATPase results in the activation of various intracellular signaling cascades that culminates in different types of cell death. It is known that cardenolides can induce apoptosis, autophagy, anoikis, and immunogenic cell death depending on the cell type and steroid chemical features [21,22,23].
Strophanthidol (4a) glycosides have been isolated from various natural sources, and are among the most potent cardenolide-based anticancer agents with the in vitro activity in low nM range for a variety of different human cancer cells (cf. Figure 1b) [24,25,26]. The sugar moiety of these compounds plays an important role in defining their anticancer activity, and without the sugar moiety, aglycone 4a is significantly less active. In addition to α-L-rhamnoside 4b, other potent strophanthidol glycosides such as 4c have been identified and investigated. These observations as well as other prior studies [27,28,29] suggest that further optimization of the sugar moiety of strophanthidol (4a) glycosides may lead to even more potent analogs. However, the glycosylation of polyoxygenated cardiotonic steroids is not trivial, primarily due to the regioselectivity issues. In most syntheses of cardiac glycosides, multistep protection/deprotection sequence is commonly used to ensure the selective delivery of a sugar unit at the desired C3 position.
This is certainly the case for the C19-hydroxylated steroids such as strophanthidol (4a), sarmentologenin (3), or ouabagenin (1) [30,31,32,33,34,35]. The primary C19-hydroxyl functionality of such steroids can undergo competitive glycosylation in the presence of the unprotected C3 hydroxyl, and the products of C19 glycosylation (i.e., 9, Scheme 1) or bis-glycosylation (i.e., 6 and 10, Scheme 1) are typically observed [30, 34]. To circumvent this, strategies requiring C19 protection, glycosylation and following deprotection have been developed [34, 35]. However, these strategies require careful selection of the C19 protecting group as the competitive decomposition of the butenolide moiety can be observed upon deprotection [34]. Altogether a more efficient strategy for the direct introduction of the sugar moiety at the C3 position of these molecules is highly desired. To streamline the synthesis of glycosylated strophanthidol (4a) analogs as well as other C3-glycosylated cardiotonic steroids possessing C3/C5/C19 hydroxylation pattern, we set out to develop a direct single pot method that is based on the use of boronic acid esters as the traceless protecting group.
The key design element present in our new protocol is the use of boronic acid as the transient protecting group for the C5/C19 diol moiety. One of the biggest advantages of using boronic acid as a protecting group is the operational simplicity and mild conditions associated with the installation and removal of the boronic acid esters. Madsen, Kaji, Taylor, and others have previously shown the utilities of various aryl boronic acids as protecting groups in the synthesis of polysaccharides [36,37,38,39]. Beyond the synthesis of simple carbohydrate derivatives, boronic acid has not been widely used to introduce sugar moiety into natural products. One of the few examples was reported by Pis and coworkers in 1994 (Scheme 2) [40]. In their synthesis of 20-hydroxyecdysone glycosides, phenylboronic acid was used to selectively protect the vicinal-diol moiety of the natural product 11 to provide ester 12. Thus formed phenylboronic acid ester was isolated and subjected to glycosylation resulting in the mixture of products 13 and 14 with intact phenylboronic acid ester. Recently, our group developed a one-pot regioselective glycosylation procedure that allows selective glycosylation of the C11 hydroxyl of 14-membered macrolactone such as 6-deoxy-erythronolide B (6-dEB) (15) [41]. The solution of 6-dEB was treated with phenylboronic acid that served as the transient protecting group for the 1,3-diol moiety of the macrolactone. The in situ formation of the boronic acid ester 16 was followed by the addition of the glycosyl donor and promoter that provided glycoside 17, and the subsequent aqueous work up was used to cleave the boronic acid ester and provide the C11 glycosylated product 18 in a single operation. Having long-term interests in improving the synthesis of cardiotonic steroids [34, 41,42,43,44,45] and building on these prior studies, we surmised that this strategy might be particularly useful for the glycosylation of steroids 1–4a, and, in particular, strophanthidol (4a). This molecule possesses hydroxyl group at the C3, C5, C14, and C19 positions, and the boronic acid protection could result in two isomeric products protected either at the C3 and C5 positions (exemplified by compound 19) or at the C5 and C19 positions (exemplified by compound 20). Our computational studies indicate that 20 is 2.6 kcal/mol more stable than 19 (DFT, B3LYP, 6–31 G*), and therefore the application of the traceless protection/glycosylation strategy to strophanthidol (4a), should lead to the selective formation of the C3 glycosides. In this paper, we describe the development of a single step protocol to directly generate unprotected C3 glycosides of strophanthidol using methylboronic acid as a transient protecting group.
Results and discussion
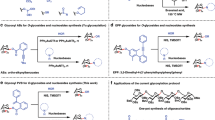
Our studies commenced with identifying the conditions for strophanthidol protection with methylboronic acid and evaluating the regioselectivity of this reaction. We surmised that methylboronic acid would preferably form cyclic ester with the C5 and less sterically hindered C19-hydroxyl group based on both the thermodynamic (vide supra) and kinetic reasons. The methylboronic acid was selected due to its lower stability of the corresponding ester toward hydrolysis in comparison to phenylboronic acid that is more commonly employed for the protection of 1,3-diols. Indeed, after we mixed strophanthidol (4a) with methylboronic acid in the presence of 4 Å molecular sieves in dichloromethane, we were able to obtain a single compound (21) containing methylboronic ester moiety with the quantitative mass recovery after a simple filtration through Celite® (Scheme 3). We subsequently subjected thus isolated boronic ester intermediate 21 to the glycosylation condition with benzyl protected fucose trichloroacetimidate donor 22 and TMSOTf as activator. Upon work up with MeOH to remove the methylboronic acid masking group, we observed exclusive glycosylation at the C3 position to form glycoside 23, albeit with moderate yield and low diastereoselectivity (57% yield, 1:2.5 d.r.). This result supports our initial hypothesis that the formation of cyclic boronate at the C5 and C19-hydroxyl groups of strophanthidol is favored exclusively. Remarkably, the less reactive tertiary C14 hydroxyl did not undergo glycosylation under these conditions. However, the glycosylation yield and diastereoselectivity had to be optimized, and the traceless protection/glycosylation/deprotection sequence had to be executed in a single operation without isolating the intermediates such as 21.
To control the diastereoselectivity, we switched the protecting group on the glycosyl donor from benzyl to benzoate, which is a well-known participating group that allows to direct the glycosylation reactions (cf. Table 1). After the in situ formation of the protected strophanthidol 21, L-rhamnose-derived glycosyl donor 7 and reaction promoter were added to the same reaction vessel to initiate the glycosylation reaction. We tested several general conditions for the activation of trichloroacetimidate donor 7, and we discovered that some of the common Lewis acid activators were inefficient in promoting the glycosylation reaction leading to 24 (entry 1–3). When TfOH was used as the activator, we were able to obtain significantly better conversion (57%) and isolated yield (37%) for the C3-glycosylated product 24. (entry 4). We also noticed that the isolated yield was increased two-fold, from 37 to 76%, when the glycosyl donor 7 was added to the reaction portionwise at 30 min intervals (entry 5). Through further optimization, we were able to obtain 24 in 81% yield exclusively as the α-diastereomer (entry 6).
To further enhance the efficiency of our protocol, we optimized the reaction work up to accomplish the deprotection of both the methylboronic ester and benzoate protecting groups on the sugar at the end of the reaction (cf. Scheme 4). After the glycosylation leading to glycosylated steroid was completed as judged by TLC, the reaction was diluted with MeOH, and the solution was saturated with ammonia by bubbling NH3 gas through it. The resultant reaction mixture was left stirring at room temperature overnight, concentrated, and purified by flash chromatography on silica gel. This simple single pot protocol resulted in unprotected strophanthidol-3-O-α-L-rhamnoside (4b) in 73% yield (entry 1).
Using this optimized procedure, we also synthesized strophanthidol glycoside 26a and 26b with unprotected L-fucose and D-fucose at the C3 position in 77 and 72% yield correspondingly (entries 2 and 3). However, when we applied this strategy to generate the D-glucose and D-mannose derivatives 26c and 26d, we noticed significant formation of the ortho-ester by-products. The formation of these side-products significantly reduced the overall yield and complicated the purification of 26c and 26d. However, we surmised that this undesired side-reaction might be easily circumvented without changing our strategy if the acyl groups that are used for the protection of sugar donors are slightly modified. In particular, Kihlberg and coworker [46] have noticed that the use of fluorine-substituted benzoates as the protecting groups on sugar donors could significantly minimize the formation of ortho-esters during the glycosylation. Indeed, when we used 2-fluorobenzoate protected D-glucose and D-mannose trichloroacetimidate donors (24c and 24d) in our reaction, the formation of ortho-esters was suppressed, and glycosides 26c and 26d were formed in 75 and 69% yield correspondingly. All in all, our one-pot protocol has allowed us to efficiently and rapidly generate five glycosylated derivatives of strophanthidol, three of which are new glycosides (26a, 26b, and 26d) that have not been reported in the literature to date.
In summary, an efficient regioselective glycosylation of strophanthidol (4a) to generate five different unprotected glycosides in a single pot (77–69% yield) has been developed. This transformation was enabled by development of regioselective protection of the C5/C19 diol moiety with methylboronic acid. This method could be generally applied to regioselective glycosylation of other C19-hydroxyl containing steroids, and the use of traceless boronic acid protection to accomplish regioselective glycosylation of steroids 1–3 is the subject of ongoing studies in our laboratories.
Experimental section
General methods
All reagents and solvents were purchased from Sigma-Aldrich, Fisher Scientific, and were used as received without further purification unless specified. Strophanthidin was purchased from Glentham Life Sciences Limited. 4 Å molecular sieve was activated prior to use by heating under reduced pressure. Cooling was achieved by use of cryocool machine or ice bath. Deionized water was used in the preparation of all aqueous solutions and for all aqueous extractions. Solvents used for extraction and chromatography were ACS or HPLC grade. Purification of reactions mixtures was performed by flash chromatography using SiliCycle SiliaFlash P60 (230–400 mesh).
1H NMR spectra were recorded on Varian vnmrs 700 (700 MHz), Varian vnmrs 500 (500 MHz), Varian INOVA 500 (500 MHz), or Varian MR400 (400 MHz) spectrometers and chemical shifts (δ) are reported in parts per million (ppm) with solvent resonance as the internal standard (CDCl3 at δ 7.26, CD3OD at δ 3.31, and C6D6 at δ 7.16). Data are reported as (br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet; coupling constant(s) in Hz; integration). Proton-decoupled 13C NMR spectra were recorded on Varian vnmrs 700 (700 MHz), Varian vnmrs 500 (500 MHz), Varian INOVA 500 (500 MHz), or Varian MR400 (400 MHz) spectrometers and chemical shifts (δ) are reported in ppm with solvent resonance as the internal standard (CDCl3 at δ 77.16, CD3OD at 49.0, C6D6 at 128.06). High resolution mass spectra (HRMS) were recorded on Micromass AutoSpec Ultima or VG (Micromass) 70–250-S Magnetic sector mass spectrometers in the University of Michigan mass spectrometry laboratory. Infrared (IR) spectra were recorded as thin film on a Thermo-Nicolet IS-50 spectrometer. Absorption peaks were reported in wavenumbers (cm−1). Optical rotations were measured in a solvent of choice on a JASCO P-2000 or Autopol III digital polarimeter at 589 nm (D-line).
Strophanthidol (4a)
Strophanthidin (500 mg, 1.24 mmol) was dissolved in EtOH (0.15 M) and cooled on an ice bath. NaBH4 (95 mg, 2.0 equiv.) was added, and the reaction was warmed up to room temperature. After stirring at room temperature for 2 h, the reaction was cooled in ice bath, diluted with 50 mL of EtOAc, and quenched slowly with 50 mL of saturated NH4Cl (aq). The biphasic mixture was stirred vigorously overnight. Next, the organic layer was separated and concentrated under reduced pressure. The crude mixture was purified by flash column chromatography on silica gel (MeOH:CH2Cl2 15:1 to 10:1 v/v) to obtain strophanthidol 4a (430 mg, 85% yield) as white solid.
Mass Spectrometry; HRMS m/z 429.2247 (M + Na, C23H34O6).
Infrared Spectroscopy; IR vmax (film) cm−1 3323, 2939, 1779, 1731, 1619, 1450, 1025.
Optical Rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 30.6 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3): δ 5.87 (1 H, s), 5.75 (1 H, s), 4.97 (1 H, d, J = 18.2 Hz), 4.80 (1 H, d, J = 17.9 Hz), 4.70 (1 H, d, J = 7.5 Hz), 4.37 (1 H, d, J = 11.2 Hz), 4.20 (1 H, d, J = 4.1 Hz), 4.07 (1 H, d, J = 4.6 Hz), 3.47 (1 H, dd, J = 11.4, 7.5 Hz), 2.76 (1 H, dd, J = 9.8, 5.5 Hz), 2.55 (1 H, td, J = 14.7, 4.1 Hz), 2.15 (1 H, dt, J = 13.6, 10.0 Hz), 2.09 (1 H, dd, J = 15.0, 3.1 Hz), 2.05–1.98 (1 H, m), 1.97–1.91 (3 H, m), 1.86 (1 H, dtd, J = 14.4, 9.3, 5.4 Hz), 1.79–1.63 (4 H, m), 1.64–1.45 (5 H, m), 1.41–1.31 (2 H, m), 1.25–1.17 (2 H, m), 0.85 (3 H, s). 13C NMR (176 MHz, CDCl3) δ 174.8, 174.7, 117.9, 85.5, 78.2, 73.7, 67.9, 65.7, 50.7, 49.6, 42.3, 40.6, 40.2, 39.0, 36.9, 35.5, 32.7, 27.7, 26.9, 24.0, 21.4, 19.2, 15.9.
Boronate 21
4a (8.0 mg, 0.020 mmol), MeB(OH)2 (1.3 mg, 1.1 equiv.), and 30 mg of powered 4 Å molecular sieve were stirred in 100 μL of dry dichloromethane at room temperature. After 2 h, the reaction was filtered through Celite and concentrated under reduced pressure to obtain boronate intermediate 21 ( > 98% mass recovery).
Infrared spectroscopy; IR vmax (film) cm−1 3470, 2938, 1780, 1737, 1620.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 21.8 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3) δ 5.88 (1 H, s), 4.96 (1 H, d, J = 18.0 Hz), 4.79 (1 H, d, J = 18.1 Hz), 4.28 (d, 1 H, J = 11.7 Hz), 4.06–3.98 (1 H, m), 3.59 (d, 1 H, J = 8.3 Hz), 3.45 (d, 1 H, J = 11.6 Hz), 2.77 (dd, 1 H, J = 9.7, 5.5 Hz), 2.20–2.15 (1 H, m), 2.10–2.02 (4 H, m), 1.95 (dd, 1 H, J = 13.9, 3.6 Hz), 1.88 (dtd, 1 H, J = 14.6, 9.4, 5.4 Hz), 1.79–1.60 (10 H, m), 1.59–1.52 (2 H, m), 1.45–1.34 (3 H, m), 1.22 (dtd, 1 H, J = 26.4, 13.6, 12.5, 5.8 Hz), 1.10 (qd, 1 H, J = 13.9, 13.4, 3.8 Hz), 0.86 (3 H, s). 13C NMR (176 MHz, CDCl3) δ 174.6, 174.3, 118.0, 85.3, 76.2, 73.6, 66.7, 65.3, 50.6, 49.6, 41.0, 40.2, 38.6, 38.1, 36.9, 36.8, 32.9, 27.6, 27.0, 23.8, 21.4, 19.8, 15.9. 10B NMR (75 MHz, CDCl3): δ 32.6 (br).
Glycoside 23
21 and trichloroacetimidate donor 22 (23.2 mg, 2.0 equiv.) was dissolved in 400 μL dry dichloromethane and cooled to –20 °C. TMSOTf (0.18 μL, 0.05 equiv.) in 20 μL dry dichloromethane was added to the reaction. After stirring for 16 h, the reaction was quenched with Et3N at room temperature and filtered through a short pad of Celite. The crude mixture was quenched and then azeotroped with MeOH multiple times and then subsequently purified by flash column chromatography on silica gel (EtOAc:Hexanes, 70:30 v/v) to obtain glycoside 23 as a mixture of two diastereomers (9.4 mg, 57% yield). The diastereomeric glycosides were separated by semi-prep HPLC (18 mL/min, EtOAc:Hexanes, 70:30 v/v) and characterized.
β-23
Mass spectrometry: HRMS m/z 845.4232 (M + Na, C50H62O10) 845.4235.
Infrared spectroscopy; IR (film) cm−1 3441, 2935, 1780, 1742, 1620, 1453, 1063.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 21.3 (c 0.31, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3) δ 7.38–7.27 (15 H, m), 5.87 (1 H, s), 5.01–4.93 (2 H, m), 4.87–4.81 (2 H, m), 4.79 (1 H, dd, J = 18.1, 1.8 Hz), 4.77–4.71 (2 H, m), 4.70 (1 H, d, J = 11.8 Hz), 4.67 (1 H, s), 4.38 (1 H, d, J = 7.7 Hz), 4.30 (1 H, d, J = 11.4 Hz), 4.24 (1 H, d, J = 10.2 Hz), 4.22 (1 H, s), 3.81 (1 H, dd, J = 9.7, 7.7 Hz), 3.57 (1 H, d, J = 2.9 Hz), 3.53 (1 H, dd, J = 9.7, 2.9 Hz), 3.50–3.43 (1 H, m), 3.42 (1 H, t, J = 10.8 Hz), 2.76 (1 H, dd, J = 9.7, 5.4 Hz), 2.54 (1 H, td, J = 14.8, 3.8 Hz), 2.19–2.10 (2 H, m), 2.07–1.96 (2 H, m), 1.95–1.82 (3 H, m), 1.76–1.71 (1 H, m), 1.70–1.62 (2 H, m), 1.60–1.44 (6 H, m), 1.39–1.24 (6 H, m), 1.20 (1 H, dd, J = 13.7, 4.5 Hz), 1.16 (3 H, d, J = 6.4 Hz), 0.85 (3 H, s). 13C NMR (176 MHz, CDCl3) δ 174.5, 174.3, 138.5, 138.4, 138.3, 128.7, 128.6, 128.5, 128.4, 128.3, 127.8, 127.7, 118.0, 101.4, 85.7, 83.1, 79.1, 76.8, 75.9, 75.7, 74.9, 74.8, 73.5, 73.2, 70.9, 64.8, 50.7, 49.5, 42.6, 40.6, 40.2, 39.1, 36.2, 35.1, 32.8, 29.9, 26.8, 24.2, 23.9, 21.3, 19.1, 17.0, 15.8.
α-23
Mass spectrometry; HRMS m/z 845.4230 (M + Na, C50H62O10).
Infrared spectroscopy; IR vmax (film) cm−1 3436, 2924, 1742, 1620, 1453.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) – 27.1 (c 0.11, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3) δ 7.41–7.27 (15 H, m), 5.88 (1 H, s), 5.21 (1 H, s), 4.99–4.94 (2 H, m), 4.83–4.77 (3 H, m), 4.77–4.71 (2 H, m), 4.65 (3 H, dd, J = 17.9, 11.8 Hz), 4.36 (1 H, d, J = 11.3 Hz), 4.02 (1 H, t, J = 2.8 Hz), 4.00 (1 H, dd, J = 10.0, 3.9 Hz), 3.91–3.83 (2 H, m), 3.65 (1 H, d, J = 1.4 Hz), 3.42 (1 H, d, J = 9.7 Hz), 2.77 (1 H, dd, J = 9.7, 5.5 Hz), 2.54 (1 H, td, J = 14.5, 4.4 Hz), 2.19–1.92 (6 H, m), 1.87 (1 H, dtd, J = 14.6, 9.4, 5.5 Hz), 1.73–1.44 (8 H, m), 1.34 (2 H, m), 1.31–1.17 (4 H, m), 1.09 (3 H, d, J = 6.5 Hz), 0.86 (3 H, s). 13C NMR (176 MHz, CDCl3): δ 174.5, 174.2, 138.6, 138.6, 138.4, 128.6, 128.4, 128.4, 127.9, 127.8, 127.7, 127.7, 118.1, 96.4, 85.7, 79.4, 77.4, 77.1, 76.7, 75.6, 75.0, 74.0, 73.5, 73.1, 72.5, 67.2, 65.0, 50.7, 49.4, 42.4, 40.6, 40.3, 39.0, 35.3, 34.2, 32.8, 29.9, 26.8, 26.2, 24.2, 21.3, 19.4, 16.8, 15.8.
Glycoside 24
4a (16.3 mg, 0.040 mmol), MeB(OH)2 (2.6 mg, 1.1 equiv.) and 60 mg of powered 4 Å molecular sieve were stirred in 200 μL of dry dichloromethane at room temperature. After 2 h, 0.5 equiv. of sugar donor 7 in 1.8 mL of dry dichloromethane was added to the reaction, and the mixture was cooled in ice bath. TfOH (1.1 μL, 0.3 equiv.) in 20 μL of dry dichloromethane was added to the reaction. At 1 h interval, 0.5 equiv. of sugar donor in 200 μL of dry dichloromethane was added to reaction (repeated three times). After the last portion of sugar portion was added, the reaction was stirred in ice bath for 1 h before being warmed up to room temperature and quenched with Et3N. Next, the reaction was filtered through a short pad of silica gel/Celite and concentrated under reduced pressure. The crude mixture was quenched and azeotroped with MeOH multiple times and then purified by flash column chromatography on silica gel (EtOAc:Hexanes, 60:40 to 70:30 v/v) to obtain 24 (27.9 mg, 81% yield) as white foamy solid.
Mass spectrometry; HRMS m/z 887.3594 (M + Na, for C50H56O13).
Infrared spectroscopy; IR vmax(film) cm−1 3485, 2935, 1780, 1729, 1601, 1451.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 9.3 (c 0.57, CHCl3).
Nuclear magnetic resonance; 1H NMR (500 MHz, CDCl3) δ 8.12–8.06 (2 H, m), 8.04–7.97 (2 H, m), 7.87–7.78 (2 H, m), 7.65–7.59 (1 H, m), 7.56–7.46 (3 H, m), 7.46–7.36 (3 H, m), 7.27–7.24 (2 H, m), 5.88 (1 H, s), 5.71 (2 H, dd, J = 5.5, 2.4 Hz), 5.66–5.60 (1 H, m), 5.16 (1 H, d, J = 1.9 Hz), 4.98 (1 H, dd, J = 18.1, 1.8 Hz), 4.81 (1 H, dd, J = 18.1, 1.7 Hz), 4.40 (1 H, d, J = 11.4 Hz), 4.30–4.19 (4 H, m), 3.50 (1 H, t, J = 10.6 Hz), 2.78 (1 H, dd, J = 9.6, 5.4 Hz), 2.60 (1 H, td, J = 14.9, 3.8 Hz), 2.20 (1 H, dd, J = 15.4, 3.3 Hz), 2.18–1.95 (5 H, m), 1.88 (1 H, dtd, J = 14.3, 9.2, 5.4 Hz), 1.79 (1 H, s), 1.76–1.68 (3 H, m), 1.64 (3 H, h, J = 4.3 Hz), 1.54 (3 H, tq, J = 11.9, 3.3 Hz), 1.36 (3 H, d), 1.35–1.31 (1 H, m), 1.28–1.15 (1 H, m), 0.87 (3 H, s). 13C NMR (125 MHz, CDCl3) δ 174.6, 174.4, 165.9, 165.7, 165.5, 133.8, 133.6, 133.3, 130.0, 129.9, 129.8, 129.2, 129.1, 129.1, 128.8, 128.6, 128.4, 117.9, 97.8, 85.5, 77.4, 77.3, 76.9, 73.6, 71.3, 71.1, 70.0, 68.1, 64.9, 50.7, 49.5, 42.7, 40.5, 40.2, 39.2, 35.8, 34.6, 32.7, 29.8, 26.8, 25.5, 24.1, 21.3, 19.2, 17.8, 15.9.
General procedure for one-pot glycosylation of strophanthidol (4a)
Strophanthidol 4a (16.3 mg, 0.040 mmol), MeB(OH)2 (2.6 mg, 1.1 equiv.) and 60 mg of powered 4 Å molecular sieve were stirred in 200 μL of dry dichloromethane at room temperature. After 2 h, 0.5 equiv. of trichloroacetimidate donor 7 or 25a-d in 1.8 mL of dry dichloromethane was added to the reaction, and the mixture was cooled in ice bath. TfOH (1.1 μL, 0.3 equiv.) in 20 μL of dry dichloromethane was added to the reaction. At 1 h interval, 0.5 equiv. of trichloroacetimidate donor in 200 μL of dry dichloromethane was added to reaction (repeated 3 times). After the last portion of trichloroacetimidate donor was added, the reaction was stirred in ice bath for 1 h before being warmed up to room temperature. Next, the reaction was diluted with 3 mL of MeOH and NH3 gas was bubbled through the reaction for 30 min. After stirring at room temperature for 18 h, the reaction mixture was filtered through a short pad of silica gel/Celite and concentrated under reduced pressure. The crude mixture was purified by flash column chromatography (on pipette column) on silica gel (starting with MeOH:CH2Cl2, 5:95 v/v + 0.5% of AcOH, followed by a gradient of MeOH:CH2Cl2, 10:90 to 20:80 v/v) to obtain glycoside 4b, 26a-d.
Glycoside 4b
Compound 4b was prepared according to general procedure for one-pot glycosylation of strophanthidol (4a) in 73% yield after flash column chromatography.
Mass spectrometry; HRMS m/z (M + Na, C29H44O10) 575.2828.
Infrared spectroscopy; IR vmax (film) cm−1 3342, 2938, 1732, 1449, 1021.
Optical rotation. \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) – 3.68 (c 1.0, CHCl3).
Nuclear magnetic resonance: 1H NMR (500 MHz, CD3OD) δ 5.90 (1 H, s), 5.03 (1 H, dd, J = 18.4, 1.8 Hz), 4.92 (1 H, dd, J = 18.4, 1.8 Hz), 4.85 (1 H, d, J = 1.8 Hz), 4.19–4.11 (2 H, m), 3.79 (1 H, dd, J = 3.4, 1.7 Hz), 3.69–3.59 (3 H, m), 3.40 (1 H, t, J = 9.5 Hz), 2.86–2.83 (1 H, m), 2.26–2.09 (5 H, m), 2.03–1.94 (1 H, m), 1.94–1.85 (2 H, m), 1.84–1.65 (6 H, m), 1.58–1.38 (9 H, m), 1.29 (1 H, s) 1.26 (3 H, d, J = 6.2 Hz), 1.25–1.19 (1 H, m), 0.89 (3 H, s). 13C NMR (125 MHz, CD3OD) δ 178.3, 177.2, 117.8, 100.9, 86.4, 77.4, 75.6, 75.3, 73.8, 72.5, 72.4, 70.7, 65.2, 51.9, 50.8, 44.1, 41.5, 41.1, 40.0, 36.2, 35.9, 33.0, 27.9, 26.4, 25.0, 22.8, 20.4, 18.0, 16.3.
Glycoside 26a
Glycoside 26a was prepared according to general procedure for one-pot glycosylation of strophanthidol in 77% yield after flash column chromatography.
Mass spectrometry; HRMS m/z (M + Na, C29H44O10) 575.2828.
Infrared spectroscopy; IR vmax (film) cm−1 3348, 2940, 1733, 1666, 1624, 1448, 1022.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 14.5 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (500 MHz, CD3OD) δ 5.90 (1 H, s), 5.03 (1 H, dd, J = 18.4, 1.8 Hz), 4.91 (1 H, dd, J = 18.4, 1.7 Hz), 4.36–4.27 (1 H, m), 4.21 (1 H, s), 4.18 (1 H, d, J = 11.4 Hz), 3.69–3.62 (1 H, m), 3.60 (1 H, d, J = 1.6 Hz), 3.58 (1 H, d, J = 11.4 Hz), 3.48–3.44 (2 H, m), 2.85–2.82 (1 H, m), 2.34–2.22 (2 H, m), 2.21–2.08 (2 H, m), 2.03–1.82 (5 H, m), 1.77–1.58 (6 H, m), 1.56–1.35 (7 H, m), 1.26 (3 H, d, J = 6.4 Hz), 1.24–1.18 (2 H, m), 0.88 (3 H, s). 13C NMR (125 MHz, CD3OD) δ 178.3, 177.2, 117.9, 102.4, 86.4, 77.3, 75.6, 75.3, 75.2, 73.0, 72.3, 72.1, 65.4, 51.9, 50.8, 44.1, 41.4, 41.1, 40.0, 37.4, 36.5, 33.0, 27.9, 25.1, 24.1, 22.7, 20.1, 16.7, 16.3.
Glycoside 26b
Compound 26b was prepared according to general procedure for one-pot glycosylation of strophanthidol in 72% yield after flash column chromatography.
Mass spectrometry; HRMS m/z (M + Na, C29H44O10) 575.2829.
Infrared spectroscopy; IR vmax (film) cm−1 3354, 2940, 1733, 1666, 1624, 1449, 1024.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 12.6 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (500 MHz, CD3OD) δ 5.90 (1 H, s), 5.03 (1 H, dd, J = 18.4, 1.8 Hz), 4.91 (1 H, dd, J = 18.4, 1.7 Hz), 4.36–4.28 (1 H, m), 4.20 (1 H, d, J = 11.3 Hz), 4.17 (1 H, s), 3.64 (1 H, m), 3.61–3.54 (2 H, m), 3.49–3.44 (2 H, m), 2.85–2.81 (1 H, m), 2.32 (1 H, td, J = 14.5, 3.8 Hz), 2.22–2.09 (4 H, m), 2.03–1.81 (4 H, m), 1.80–1.61 (6 H, m), 1.58–1.34 (7 H, m), 1.26 (3 H, d, J = 6.5 Hz), 1.24–1.16 (2 H, m), 0.88 (3 H, s). 13C NMR (125 MHz, CD3OD) δ 178.3, 177.2, 117.9, 102.6, 86.4, 77.3, 75.8, 75.3, 75.3, 73.1, 72.3, 72.1, 65.6, 51.9, 50.8, 43.8, 41.4, 41.1, 40.0, 36.8, 35.3, 33.1, 27.9, 26.5, 25.2, 22.6, 20.5, 16.8, 16.3.
Trichloroacetimidate donor 25c
D-Glucose (300 mg, 1.67 mmol) and DMAP (102 mg, 0.5 equiv.) were dissolved in dry pyridine and cooled on an ice bath. 2-fluorobenzoyl chloride (1.4 mL, 7.0 equiv.) was added to the reaction dropwise. After stirring at room temperature overnight, the reaction was quenched with 2 mL of MeOH and stirred for 1 h. Next, the reaction was diluted with 50 mL of DCM and washed with 2 N HCl (two times), sat. NaHCO3 (two times), and brine solution. The organic layer was dried over Na2SO3, filtered and concentrated under reduced pressure. The crude mixture was used in next step without further purification.
The crude from previous step was dissolved in 5 mL of DCM and 33% HBr in AcOH was added to the reaction dropwise. After stirring at room temperature for 5 h, the reaction was quenched slowly with sat NaHCO3 (aq). Next, the mixture was diluted with DCM, and washed with sat NaHCO3 (aq), followed by brine solution. The organic layer was dried over Na2SO3, filtered and concentrated under reduced pressure. The crude mixture was used in next step without further purification.
The crude from previous step was dissolved in acetone/H2O/DCM (10 mL: 1 mL: 2 mL) before Ag2CO3 (800 mg) was added to the reaction. After stirring at room temperature for overnight, the reaction was filtered through Celite and concentrated under reduced pressure. The crude mixture was purified by flash column chromatography on silica gel (EtOAc: hexanes, 30:70 to 40:60 v/v) to obtain benzoate protected hemiacetal, which (300 mg, 0.45 mmol) was dissolved in 3 mL of DCM (0.15 M) and cooled on an ice bath. Trichloroacetonitrile (0.9 mL, 20 equiv.) was added to the reaction, followed by DBU (34 μL, 0.5 equiv.). After stirring at room temperature for 2 h, the reaction was concentrated under reduced pressure. The crude mixture was purified by flash column chromatography on silica gel (EtOAc:hexanes, 15:85 v/v) to obtain α-trichloroacetimidate donor 25c (250 mg, 68% yield) as white foamy solid.
Mass spectrometry; HRMS m/z (M + Na, C36H24Cl3F4NO10) 834.0301.
Infrared spectroscopy; IR vmax (film) cm−1 1729, 1677, 1612, 1584, 1489, 1456, 1290, 1243.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 66.5 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3) δ 8.67 (1 H, s), 7.96 (1 H, t, J = 7.5 Hz), 7.89 (1 H, t, J = 7.5 Hz), 7.83 (1 H, t, J = 7.4 Hz), 7.77 (1 H, t, J = 7.5 Hz), 7.54–7.48 (3 H, m), 7.44 (1 H, q, J = 7.1 Hz), 7.21 (1 H, t, J = 7.5 Hz), 7.18–7.09 (4 H, m), 7.08 (2 H, t, J = 9.5 Hz), 7.02 (1 H, t, J = 9.5 Hz), 6.24 (1 H, t, J = 9.9 Hz), 5.80 (1 H, t, J = 9.9 Hz), 5.60 (1 H, dd, J = 10.2, 3.7 Hz), 4.67–4.60 (2 H, m), 4.57 (1 H, dd, J = 12.7, 4.9 Hz). 13C NMR (176 MHz, CDCl3) δ 163.9, 163.9, 163.2, 163.2, 163.2, 163.1, 163.0, 163.0, 162.9, 162.9, 162.8, 162.8, 161.7, 161.5, 161.3, 161.3, 160.7, 135.4, 135.4, 135.2, 135.2, 134.9, 134.9, 134.9, 134.8, 132.4, 132.3, 132.1, 131.9, 124.3, 124.2, 124.2, 124.2, 124.2, 124.1, 124.1, 124.1, 118.3, 118.2, 118.0, 118.0, 117.7, 117.7, 117.3, 117.2, 117.2, 117.1, 117.1, 117.1, 116.9, 93.1, 90.8, 70.8, 70.6, 70.4, 68.9, 62.7 (complex multiplet in δ 164–117 due to C-F couplings).
Glycoside 26c
Compound 26c was prepared according to general procedure for one-pot glycosylation of strophanthidol in 75% yield after flash column chromatography.
Mass sepctrometry; HRMS m/z (M + Na, C29H44O11) 591.2791.
Infrared spectroscopy; IR vmax (film) cm−1 3341, 2941, 1734, 1668, 1450, 1019.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 10.1 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (500 MHz, CD3OD) δ 5.90 (1 H, s), 5.03 (1 H, dd, J = 18.5, 1.8 Hz), 4.95–4.87 (1 H, dd, J = 18.5, 1.7 Hz), 4.40 (1 H, d, J = 7.9 Hz), 4.23 (1 H, s), 4.20 (1 H, d, J = 11.4 Hz), 3.86 (1 H, dd, J = 11.9, 1.7 Hz), 3.69–3.63 (1 H, m), 3.58 (1 H, d, J = 11.4 Hz), 3.29–3.26 (2 H, m), 3.18 (1 H, dd, J = 9.2, 7.9 Hz), 2.85–2.82 (1 H, m), 2.32 (1 H, td, J = 14.5, 4.0 Hz), 2.22–2.09 (3 H, m), 2.05–1.81 (4 H, m), 1.81–1.59 (6 H, m), 1.57–1.32 (7 H, m), 1.32–1.20 (2 H, m), 0.88 (3 H, s). 13C NMR (125 MHz, CD3OD) δ 178.3, 177.2, 117.9, 102.0, 86.4, 78.2, 78.2, 77.3, 75.7, 75.3, 75.1, 71.6, 65.6, 62.7, 51.9, 50.8, 43.8, 41.4, 41.0, 39.9, 36.9, 35.2, 33.1, 27.9, 26.5, 25.2, 22.6, 20.5, 16.3.
Trichloroacetimidate donor 25d
Compound 25d was prepared from D-mannose following the same synthetic procedures as for 25c. Mass Spectrometry; HRMS m/z (M + Na, C36H24Cl3F4NO10) 834.0303.
Infrared spectrometry; IR vmax (film) cm−1 1725, 1678, 1612, 1585, 1488, 1456, 1289, 1243.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) – 34.3 (c 1.0, CHCl3).
Nuclear magnetic resonance; 1H NMR (700 MHz, CDCl3) δ 8.87 (1 H, s), 7.99 (2 H, q, J = 7.8 Hz), 7.82 (2 H, q, J = 7.9 Hz), 7.59–7.56 (1 H, m), 7.54–7.46 (2 H, m), 7.44 (1 H, qd, J = 7.0, 6.5, 2.8 Hz), 7.19 (1 H, t, J = 7.6 Hz), 7.16–7.11 (3 H, m), 7.11–7.05 (3 H, m), 7.01 (1 H, dd, J = 10.6, 8.4 Hz), 6.57 (1 H, s), 6.21 (1 H, t, J = 9.7 Hz), 5.97–5.95 (2 H, m), 4.69–4.65 (1 H, m), 4.62–4.56 (2 H, m). 13C NMR (176 MHz, CDCl3) δ 163.8, 163.8, 163.2, 163.2, 163.1, 163.1, 163.1, 163.0, 162.8, 161.7, 161.6, 161.6, 161.3, 159.9, 135.4, 135.3, 135.1, 135.1, 134.8, 134.7, 132.5, 132.5, 132.2, 132.1, 124.2, 124.2, 124.2, 124.1, 124.1, 124.0, 124.0, 118.4, 118.3, 117.9, 117.8, 117.7, 117.6, 117.6, 117.5, 117.3, 117.2, 117.2, 117.1, 117.1, 117.1, 117.0, 94.6, 90.7, 71.5, 70.1, 69.0, 66.4, 62.8 (complex multiplet in δ 164–117 due to C-F couplings).
Glycoside 26d
Compound 26d was prepared according to general procedure for one-pot glycosylation of strophanthidol in 69% yield after flash column chromatography.
Mass spectrometry; HRMS m/z (M + Na, C29H44O11) 591.2780.
Infrared spectroscopy; IR vmax (film) cm−1 3358, 2939, 1733, 1667, 1617, 1452, 1027.
Optical rotation; \([{\rm{\alpha}}]_{{\mathrm{D}}}^{25}\) + 46.9 (c 0.98, CHCl3).
Nuclear magnetic resonance; 1H NMR (500 MHz, CD3OD) δ 5.88 (1 H, s), 5.01 (1 H, dd, J = 18.5, 1.8 Hz), 4.94–4.86 (2 H, m), 4.24 (1 H, s), 4.14 (1 H, d, J = 11.4 Hz), 3.86–3.78 (2 H, m), 3.72–3.55 (5 H, m), 2.82–2.80 (1 H, m, J = 9.0 Hz), 2.34 (1 H, dd, J = 15.3, 3.5 Hz), 2.22–2.02 (3 H, m), 2.00–1.79 (5 H, m), 1.77–1.59 (5 H, m), 1.57–1.34 (8 H, m), 1.31–1.15 (2 H, m), 0.87 (3 H, s). 13C NMR (125 MHz, CD3OD) δ 178.3, 177.2, 117.9, 98.5, 86.4, 77.5, 75.4, 75.3, 72.8, 72.7, 72.4, 68.6, 65.2, 63.0, 51.9, 50.8, 44.3, 41.5, 41.0, 40.0, 37.7, 36.1, 33.0, 27.9, 25.0, 23.0, 22.8, 20.0, 16.3.
References
Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. Am J Physiol Heart Circ Physiol. 2005;289:H1781–H1793.
Wiesner K, Tsai TYR. Some recent progress in the synthetic and medicinal chemistry of cardioactive steroid glycosides. Pure Appl Chem. 1986;58:799–810.
Heasley B. Chemical synthesis of the cardiotonic steroid glycosides and related natural products. Chem Eur J. 2012;18:3092–120.
Michalak M, Michalak K, Wicha J. The synthesis of cardenolide and bufadienolide aglycones, and related steroids bearing a heterocyclic subunit. Nat Prod Rep. 2017;34:361–410.
Pessoa MTC, Barbosa LA, Villar JAFP. Synthesis of cardiac steroids and their role on heart failure and cancer. Stud Nat Prod Chem. 2018;57:79–113.
Ahmad VU, Basha A. Spectroscopic Data of Steroid Glycosides: Spirostanes, Bufanolides, Cardenolides.. New York: Springer; 2006. p. 524–695.
Ahmad VU, Basha A. Spectroscopic Data of Steroid Glycosides: Cardenolides and Pregnanes.. New York: Springer; 2006. p. 2089–759.
Nesher M, Shpolansky U, Rosen H, Lichstein D. The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci. 2007;80:2093–107.
Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Phsyiol Cell Physiol. 2007;293:C509–C536.
Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev. 2008;7:926–35.
Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharm Rev. 2009;61:9–38.
Babula P, Masarik M, Adam V, Provaznik I, Kizek R. From Na+/K+-ATPase and cardiac glycosides to cytotoxicity and cancer treatment. AntiCancer Agent Med Chem. 2013;13:1069–87.
Schneider NFZ, Cerella C, Simoes CMO, Diederich M. Anticancer and immunogenic properties of cardiac glycosides. Molecules. 2017;22:1932–47.
Cai H, et al. Digitoxin analogues with improved anticytomegalovirus activity. ACS Med Chem Lett. 2014;5:395–9.
Akhtar N, Malik A, Ali SN, Kazmi SU. Proceragenin, an antibacterial cardenolide from Calotropis procera. Phytochemistry. 1992;31:2821–4.
Jacob PL, et al. Immunomodulatory activity of ouabain in Leishmania leishmania amazonensis-infected Swiss Mice. Parasitol Res. 2013;112:1313–21.
Yoneyama T, et al. Notch inhibitors from Calotropis gigantea that induce neuronal differentiation of neural stem cells. J Nat Prod. 2017;80:2453–61.
Orellana AM, Kinoshita PF, Leite JA, Kawamoto M, Scavone C. Cardiotonic steroids as modulators of neuroinflammation. Front Endocrinol. 2016;7:1–10.
Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R529–35.
Mijatovic T, Kiss R. Cardiotonic steroids-mediated Na+/K+-ATPase targeting could circumvent various chemoresistance pathways. Planta Med. 2013;79:189–98.
Yang P, et al. Cellular location and expression of Na+, K+‐ATPase α subunits affect the anti‐proliferative activity of oleandrin. Mol Carcinolg. 2014;53:253–63.
Goncalves-de-Albuquerque FC, Silva RA, da Silva IC, Castro-Faria-Neto CH, Burth P. Na/K Pump and beyond: Na/K-ATPase as a modulator of apoptosis and autophagy. Molecules. 2017;22:578–96.
Simpson CD, et al. Inhibition of the sodium potassium adenosine triphosphatase pump sensitizes cancer cells to anoikis and prevents distant tumor formation. Cancer Res. 2009;69:2739–47.
Jiang M-M, et al. Cardenolides from Antiaris toxicaria as potent selective Nur77 modulators. Chem Pharm Bull. 2008;56:1005–8.
Shi L-S, et al. Cytotoxic cardiac glycosides and coumarins from antiaris toxicaria. Bioorg Med Chem. 2014;22:1889–98.
Tian D-M, Cheng H-Y, Shen W-Z, Tang J-S, Yao X-S. Cardiac glycosides from the seeds of Thevetia peruviana. J Nat Prod. 2016;79:38–50.
Langenhan JM, Peters NR, Guzei AI, Hoffmann M, Thorson JS.Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization. Proc Natl Acad Sci USA2005;102:12305–10.
Iyer AKV, et al. Direct comparison of the anticancer activities of digitoxin MeON-neoglycosides and O-glycosides. ACS Med Chem Lett. 2010;1:326–30.
Li X. et al. Synthesis of C3-neoglycosides of digoxigenin and their anticancer activities. Eur J Med Chem. 2018;145:252–62.
Pal’yants N, Sh., Abubakirov NK. Glycosylation of cardiosteroids. Khim Prir Soedin. 1984;5:515–29.
Hong B-C, Kim S, Kim T-S, Corey EJ. Synthesis and properties of several isomers of the cardioactive steroid ouabain. Tetrahedron Lett. 2006;47:2711–5.
Zhang H, Reddy MS, Phoenix S, Deslongchamps P. Total synthesis of ouabagenin and ouabain. Angew Chem Int Ed. 2008;47:1272–5.
Reddy MS, Zhang H, Phoenix S, Deslongchamps P. Total synthesis of ouabagenin and ouabain. Chem Asian J. 2009;4:725–41.
Bhattarai B, Nagorny P. Concise enantioselective synthesis of oxygenated steroids via sequential Copper(II)-catalyzed Michael addition/intramolecular Aldol cyclization reactions. Org Lett. 2018;20:154–7.
Urabe D, et al. Total synthesis and biological evaluation of 19-Hydroxysarmentogenin-3-O-α-l-rhamnoside, trewianin, and their aglycons. J Org Chem. 2018;83:13888–910.
Fenger, TH; Madsen, R Regioselective Glycosylation of Unprotected Phenyl 1-Thioglycopyranosides with Phenylboronic Acid as a Transient Masking Group. Eur. J. Org. Chem. 2013;26:5923–33.
Kaji E, Nishino T, Ishige K, Ohya Y, Shirai Y. Regioselective glycosylation of fully unprotected methyl hexopyranosides by means of transient masking of hydroxy groups with arylboronic acids. Tetrahedron Lett. 2010;51:1570–3.
Mancini RS, Lee JB, Taylor MS. Boronic esters as protective groups in carbohydrate chemistry: processes for acylation, silylation, and alkylation of glycoside-derived boronates. Org Biomol Chem. 2017;15:132–43.
Dimakos V, Taylor MS. Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem Rev. 2018;118:11457–517.
Pis J, Hykl J, Budesinsky M, Harmatha J. Regioselective synthesis of 20-hydroxyecdysone glycosides. Tetrahedron. 1994;50:9679–90.
Tay J-H, et al. Regiodivergent glycosylations of 6-deoxy-Erythronolide B and Oleandomycin-derived macrolactones enabled by chiral acid catalysis. J Am Chem Soc. 2017;139:8570–8.
Cichowicz N, et al. Concise enantioselective synthesis of oxygenated steroids via sequential copper(II)-catalyzed Michael addition/intramolecular Aldol cyclization reactions. J Am Chem Soc. 2015;137:14341–8.
Cichowicz N, Nagorny P. New strategy based on sequential Michael/Aldol reactions for the asymmetric synthesis of cardenolides. Strat Tactics Organ Synth. 2016;12:237–67.
Kaplan W, Khatri HR, Nagorny P. Concise enantioselective total synthesis of cardiotonic steroids 19-hydroxysarmentogenin and trewianin aglycone. J Am Chem Soc. 2016;138:7194–8.
Lee J, Wang S, Callahan M, Nagorny P. Copper(II)-catalyzed tandem decarboxylative Michael/Aldol reactions leading to the formation of functionalized cyclohexenones. Org Lett. 2018;20:2067–70.
Sjolin P, Kihlberg J. Use of fluorobenzoyl protective groups in synthesis of glycopeptides: β-elimination of O-linked carbohydrates is suppressed. J Org Chem. 2001;66:2957–65.
Acknowledgements
This work was supported by NIH grant R01GM111476 (PN). PN is the Sloan Foundation and Amgen Young Investigator Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedication: This article is dedicated to Professor Samuel J. Danishefsky for his great contributions to the areas of total synthesis of complex natural products and glycoscience.
Rights and permissions
About this article
Cite this article
Tay, JH., Dorokhov, V., Wang, S. et al. Regioselective single pot C3-glycosylation of strophanthidol using methylboronic acid as a transient protecting group. J Antibiot 72, 437–448 (2019). https://doi.org/10.1038/s41429-019-0172-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0172-1