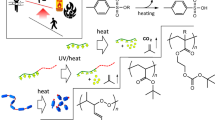
Abstract
Photoinduced transitions between the solid, glass, and liquid states based on molecular photoswitches promise a wide variety of applications. Photoswitchable adhesives are representative examples and are expected to contribute to material recycling for a sustainable future in the era of composite materials due to strong bonding and on-demand photo-induced debonding with minimal damage to the adherends. Only a few molecular photoswitches are known to undergo these transitions, but recent progress, mainly with azobenzene, has been remarkable. Here, we review the photoinduced transitions of small molecules and polymers over approximately a decade and systematically discuss the molecular designs, mechanisms, applications, merits and demerits, and future challenges in each photoswitch and the whole field. We hope this review provides useful information, inspiration, and ideas for the development of this field and the expansion of its applications.
Similar content being viewed by others
Introduction
Transitions between solids (crystals and some elastomers with crystalline or glassy regions), glasses (frozen liquids), and flowing liquids at room temperature induced by photoirradiation and resulting changes in the melting points (Tms) and glass transition temperatures (Tgs) [1,2,3,4] have attracted considerable attention in various fields and are considered for applications such as photoswitchable adhesives [5, 6], energy storage and release [7,8,9,10,11,12,13], photolithography [14], actuators [15], healable materials, and gas separations (Fig. 1a). Unlike conventional temperature control to induce the transitions, isothermal photostimulation is fascinating because of the high spatiotemporal resolution, easy and precise regulation of the wavelength and intensity, and no generation of chemical waste. In particular, photoswitchable adhesives are expected to contribute to material recycling for a sustainable future as complex architectures composed of dissimilar materials are being developed in diverse fields [16,17,18,19,20], because they enable both robust bonding during use and on-demand debonding after use with minimal damage to the adherends due to the advantages of light. In addition to photoinduced transitions, polarity changes [21,22,23,24,25,26] and bond formation and dissociation [27,28,29,30,31,32,33,34,35,36] (and resulting liquid crystal–liquid transitions [37]) have enabled the photocontrol of adhesion [5]. The photoinduced transitions of small molecules and polymers have been achieved with molecular photoswitches, which are (thermo)reversibly isomerized between at least two thermodynamically (meta)stable states with photoirradiation [38,39,40,41,42]. Although a huge variety of molecular photoswitches are known and widely used, to the best of our knowledge, only five of them have been demonstrated to show photoinduced transitions, and these are azobenzene (AB), spiropyran (SP), hydrazone, diarylethene (DAE), and 1,2-diketone (Fig. 1b), since the first paper using AB was reported in 2011 [43]. Moreover, the previously published papers were predominantly on AB. The progress realized primarily with AB small molecules and polymers has been summarized in excellent reviews [1,2,3,4]. In this article, we highlight recent progress in photoinduced transitions based on AB and other photoswitches. Transitory liquefaction induced by the photothermal effect or continuous photoisomerization cycles and photoinduced transitions of liquid crystals [44,45,46,47,48,49,50,51,52,53,54], between sols and gels, and between liquids and elastomers without crystalline or glassy regions are beyond the scope of this review. Here, previous studies on photoinduced transitions between solids, glasses, and liquids are divided into eight sections based on the kinds of photoswitches and molecular weights, i.e., small molecules or polymers including monodisperse oligomers and dendrimers, and discussed separately in terms of the molecular designs, mechanisms, applications, merits and demerits, and future challenges. Finally, we also suggest future challenges for the whole field.
Azobenzene small molecules
The photoinduced transitions based on AB are described in the other remarkable reviews [1,2,3,4]. Here, we will describe and discuss only the essence of the previous studies. AB is definitely the most commonly used molecular photoswitch and is characterized by large motions generated during isomerization between the thermodynamically stable E isomer and the metastable Z isomer during irradiation with UV and visible light (Fig. 1b) [55, 56]. Macrocyclic AB molecules with long alkyl chains were discovered for the first time to show photoinduced solid–liquid phase transitions at room temperature (Fig. 2a) [43]. Both the dimer and trimer macrocycles, in which all incorporated ABs were the E isomers, were isothermally changed from the crystalline to the isotropic phase by UV light (Fig. 2b, c). The trimer changed more slowly than the dimer, and the corresponding macrocycles without long alkyl chains did not undergo these photoinduced transitions. The phase transitions were highly sensitive to light and induced even by the generation of a small Z fraction. One reason for the high sensitivity is that E/Z mixtures generally have much lower Tms than the pure E and Z isomers, as observed with the parent AB [57, 58], which is significantly different from unidirectional Tg changes in AB-containing polymers as a function of the E/Z ratio. Subsequent work demonstrated that the macrocyclic E/E dimer isomerized to the E/Z isomer to the Z/Z isomer under UV irradiation and underwent transitions from crystalline to liquid to another crystalline phase, which were reversibly induced with visible light exposure and heating (Fig. 2d) [59]. The photoinduced liquefaction mechanism was also elucidated by X-ray crystallography under UV irradiation at different temperatures [60]. After the first paper, photoinduced transitions from a crystal to a liquid and between crystals, glasses, and liquids were reported for an asymmetric ortho-alkylated AB [61] and sugar alcohol derivatives with multiple ABs [62], respectively (Fig. 2e, f). Although the asymmetric AB has not been studied subsequently, a systematic study on a series of multi-AB sugar alcohol derivatives was conducted and found that those with one or two ABs did not isomerize and liquefy upon UV irradiation, probably due to the high crystallinity and absence of available free volume for isomerization [63,64,65]. On the other hand, the syntheses and isolation of these AB derivatives were relatively difficult. Therefore, since then, most of the molecular structures have been simple but asymmetric and composed of one AB and one or more long alkyl chains. A typical example is shown in Fig. 2g [66,67,68,69,70]. Two alkyl chains were added at the (para) 4- and 4’-positions of AB, and one methyl group was introduced at the (meta) 3-position. Generally, long alkyl chains prompt easy E-AB aggregation and make Z-AB a liquid due to the large free volume [65]. The methyl group broke the molecular symmetry, disrupted crystal packing, destabilized the crystal structures, provided enough free volume for photoisomerization, and enabled photoinduced crystal–liquid transitions. The corresponding symmetric AB derivatives without the methyl group or with two methyl groups at the 3- and 3’-positions underwent no photoinduced transitions. In addition to these AB small molecules, a wide variety of ABs have shown photoinduced crystal–liquid transitions, including an amphiphile [71], ionic crystals and liquids [72,73,74], star-shaped tetramers [75, 76], simple ABs with one or two small substituents such as methyl and methoxy groups [77,78,79,80,81,82,83], and others [84,85,86,87].
a Macrocyclic AB molecules reported in the first paper. Photoinduced transition from b crystal to c liquid states of macrocyclic AB (n = 1) and d the details. Reproduced with permission from Norikane et al. [43]. Copyright 2011, the Royal Society of Chemistry. e An asymmetric ortho-alkylated AB, f sugar alcohol derivatives with multiple ABs, and g a typical example of simple asymmetric ABs that show photoinduced transitions between crystal, glass, and liquid states. Reproduced with permission from Akiyama and Yoshida [62]. Copyright 2012, John Wiley & Sons. h Visible and NIR light-responsive ortho-substituted, coordinated, and bridged ABs. i Representative thermally stable azoheteroarenes. j A photoswitchable azoheteroarene adhesive. Reproduced with permission from Huang et al. [120]. Copyright 2022, American Chemical Society
The simple AB skeleton was employed in all molecules described above but has two fatal drawbacks for some applications, i.e., UV excitation is required for E-to-Z isomerization, and the Z isomer has a short half-life (t1/2) of approximately 1 day [55, 56]. UV light causes damage to organic and polymeric materials and biological components, although the high spatiotemporal resolution of the light can minimize the effect. The Z isomer formed the liquid state with high molecular mobility and therefore could not maintain the state due to the short t1/2 in the photoinduced transitions described above. To broaden the application range, the excitation wavelength has been redshifted to the visible and near infrared (NIR) regions mainly by ortho-substitution, coordination, and bridging of the two phenyl rings (Fig. 2h) [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103], and the thermal stability of the Z isomer has been improved to provide t1/2 values of up to several decades (at 25 °C in solution) for ortho-substituted ABs and azoheteroarenes (Fig. 2i) [104,105,106,107,108,109,110,111,112]. Recently, some of them have been demonstrated to show crystal–liquid transitions with only visible light [113, 114] and by photoisomerization with a thermally stable Z isomer [106, 115,116,117,118,119,120,121,122]. Photoinduced transitions between liquid E-AB and solid (crystalline) Z-AB would suppress thermal isomerization by restricting the molecular mobility of the Z isomer as approached in polymers (Fig. 3c), but this has not been reported. The Z isomer of the parent AB has a higher Tm (ca. 71 °C) than the E isomer (ca. 68 °C) [3, 4, 123], but modification of the AB skeleton reverses the original Tms. The reason is still unknown [3].
a Representative poly(meth)acrylate linear homopolymers of AB that show photoinduced transitions between glass and liquid states. b Photoinduced glass–liquid transitions of an AB homopolymer (R1 = H, m = 6, R2 = CH3). c Photoinduced transitions between the liquid E isomer and the physically cross-linked solid-like Z isomer using an AB monomer fused with a quadruple hydrogen bonding ureidopyrimidinone moiety. d Healing of the AB homopolymer bulk material with UV and visible light irradiation (R1 = H, m = 6, R2 = CH3). Reproduced with permission from Zhou et al. [132]. Copyright 2017, Springer Nature
To date, photoinduced transitions of AB small molecules have been used for switching fluorescence [61] and enzymatic degradation of a biodegradable polymer [68], switchable adhesives [62,63,64,65, 67, 115, 120, 122], photoresists for simple photolithography without harsh conditions [66, 74, 119], a remote-controllable light shutter [71], energy storage and release [72, 74, 76, 80, 82,83,84, 86, 87, 113, 114, 116,117,118, 121], gas separation [75], moving crystals on glass and water surfaces [77,78,79, 81], an actuator [69], and shape memory [70]. A representative example of photoswitchable adhesives is shown in Fig. 2j. An azoheteroarene with a t1/2 of several days and almost quantitative photoisomerization yields in both E–Z directions was used as the adhesive and showed reversible bonding via solidification and debonding via liquefaction induced by visible and UV irradiation, respectively. Photoisomerization was clearly confirmed by the color changes. Nevertheless, incorporation of the system in polymers is desired because commercial adhesives are mainly composed of polymers. In energy storage and release, blending small ABs with photoinactive phase-change materials [124,125,126,127,128,129,130] or solely unliquefiable ABs [131] is a powerful strategy.
Azobenzene polymers
Photoinduced transitions between solid, glass, and liquid states of polymers were independently reported for the first time in 2017 by two research groups [132, 133]. Both pioneering studies demonstrated that glassy linear polyacrylates with E-AB in the side chains were converted to flowing liquids with Tgs below room temperature upon UV irradiation and the consequent E-to-Z isomerization and returned to glasses by visible irradiation and Z-to-E isomerization (Fig. 3a, b). The Tgs monotonically decreased with increasing Z ratios [132], which was completely different from the dependence of Tms on the E/Z ratios in AB small molecules. The effect of molecular weight on photoliquefaction was also investigated [132, 134]. Interestingly, all polyacrylate linear homopolymers of AB (R1 = H, m = 6, R2 = CH3 in Fig. 3a) with different molecular weights ranging from 5 × 103 g mol−1 to 105 g mol−1 were photoliquefied. The changes in Tgs between the original E polymer and a photostationary state (PSS) in the E-to-Z isomerization (ΔTg) were similar. Rather, the ΔTg in the 105 g mol−1 polymer (ca. 70 °C) was larger than that with a molecular weight of 104 g mol−1 (ca. 60 °C), although the higher molecular weight resulted in increased Tgs for both isomers (E: from 48 °C to 80 °C, Z: from −10 °C to 7 °C). A high molecular weight also generated bulk physical properties such as free-standing and stretchable capabilities. Regarding the polymer structure, the length of the alkyl chain spacer, by which AB was connected to the main chain, should not be too short or too long [135]. Short and long spacers failed to induce phototransitions due to the restricted molecular mobility of AB and the crystalline natures of long alkyl chains such as n-C20H40, respectively. Similarly, the length of the alkyl chain at the side chain end significantly affected the photoinduced transitions [136]. Longer tails up to n-C16H33 resulted in higher Tgs for both isomers but larger ΔTg and lower viscosity for the Z isomers. Moreover, polyacrylate was more appropriate than polymethacrylate as the main chain backbone for photoinduced glass–flowing liquid transitions due to its higher flexibility [137, 138]. In addition to the side chains of these poly(meth)acrylate linear homopolymers [122, 132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147], AB has been incorporated into a variety of polymers and positions to enable photoinduced transitions between solids, glasses, and liquids, including side chains of other linear homopolymers (poly(vinyl ether) and polynorbornene) [148, 149], linear statistical copolymers [150, 151], and linear and star block copolymers [152,153,154,155], main chains of linear polymers [156,157,158,159,160], both chain ends of linear oligomers [161, 162], one end of a linear polymer [163], cross-linked polymers [164, 165], and the peripheries of dendrimers [166].
Photoinduced transitions were also demonstrated for azoheteroarenes [122, 141], with only visible light [157], and for crystalline polymers [140, 147, 148, 155,156,157,158, 161,162,163]. Furthermore, photoinduced transitions between a stable liquid E isomer and a physically cross-linked solid-like metastable Z isomer were achieved by fusing AB with a quadruple hydrogen-bonding ureidopyrimidinone moiety and incorporating it into the side chains of liquid polysiloxanes (Fig. 3c) [151]. The reverse direction to the common transitions using AB could solve the thermal isomerization and back transition occurring at room temperature by restricting the molecular mobility of the Z isomer, which has never been addressed in AB small molecules and promises to broaden the applications. Meanwhile, the intrinsic instability of Z-AB was not improved (t1/2 = ca. 8 h at 25 °C in solution), and this system may be excluded from the scope of this review because the Tms and Tgs were not determined.
The number of papers on photoinduced transitions of AB-containing polymers is still small compared to that of low-molecular-weight ABs. In particular, the use of upgraded ABs [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] and azoheteroarenes [104,105,106,107,108,109,110,111,112] is rare but needed. In terms of practical use, blending AB small molecules into photoinactive polymers is an effective strategy to realize photoinduced transitions of the polymeric materials [167,168,169,170,171,172]. To date, photoinduced transitions of polymers have been considered for applications including healable materials (Fig. 3d) [132, 134,135,136, 138, 160, 163, 166, 170], switchable adhesives [122, 133, 137, 139, 148, 150,151,152,153,154, 159,160,161, 166, 168, 169], actuators [134, 157, 158, 164, 165], switching thermal conductivity [140, 147] and fluorescence [172], fabrication of micro- and nanopatterns (lithography) [142, 144, 149, 155, 160], information encryption and smart windows [163], shape memory [165], and energy storage and release [166].
Spiropyran small molecule
Noncharged SP isomerizes to the metastable zwitterionic merocyanine (MC) under UV irradiation and spontaneously reverts back to SP at room temperature and more rapidly with heating or visible irradiation (Fig. 1) [173, 174]. The large polarity changes are intriguing and have been utilized in various applications [21, 22, 24,25,26, 175]. To date, only one SP small molecule has been reported to show photoinduced solid–liquid transitions [176]. An SP derivative with two long alkyl chains was a liquid but changed to a solid upon UV light exposure and subsequent thermal treatment (Fig. 4a, b). The J-aggregate of MC and blue crystals were formed by heating at 30 °C, while the H-aggregate and red crystals were generated by heating at 35–55 °C. Heating above the estimated Tms of the crystals (> 60 °C) or visible light exposure resulted in liquefaction and color fading. In contrast to the photoinduced transitions based on AB, the thermally unstable MC isomer formed a solid. Therefore, both crystals were stable, and no thermal isomerization to SP was observed in the dark at room temperature.
a An SP small molecule that shows photoinduced solid–liquid transitions and b the details. c Linear statistical copolymers containing SP with photoswitchable Tgs. d A fluorescent hydrazone and e its photoinduced crystal–isotropic transitions (left: fluorescence images, right: polarizing images). Reproduced with permission from Shao et al. [186]. Copyright 2018, American Chemical Society. f, g Acyclic and cyclic hydrazones that change their Tms upon light exposure. h Linear homopolymers of hydrazone that show photoinduced Tg changes. i Formation and disappearance of microfibrils on DAE crystals via photoinduced crystal-to-liquid transitions. Reproduced with permission from Uchida et al. [191]. Copyright 2006, John Wiley and Sons. j Photoinduced crystal-to-liquid transition of an asymmetric heteroaromatic 1,2-diketone. Reproduced with permission from Komura et al. [193]. Copyright 2023, the Royal Society of Chemistry
Spiropyran polymers
Switching of Tgs by SP–MC photoisomerization has been achieved with linear polymers and a cross-linked polymer [177, 178], although photoinduced transitions between glasses and flowing liquids have not been demonstrated at room temperature. The Tgs of the linear statistical copolymers of an SP methacrylate and di(ethylene glycol)methyl ether methacrylate with different compositions were increased by up to ca. 20 °C upon UV irradiation, probably due to the high polarity of the generated MC isomer and consequent enhanced intermolecular interactions (Fig. 4c) [178]. The direction of the Tg changes was opposite to that of AB polymers and similar to the Tm changes in the SP small molecule. However, unlike the small SP, the MC form in the bulk reverted back to the SP form at room temperature over a period of 2–3 h. In the cross-linked polymer, the Tg increase seen during UV light exposure was ca. 10 °C and smaller than those of the linear polymers, but some fraction of the MC form was maintained for several months in the dark [177]. Photoinduced glass–liquid transitions at room temperature will be possible by optimization of the compositions in both systems.
Hydrazone small molecules
Recently, hydrazone photoswitches with extremely high thermal stabilities have been developed [179,180,181,182,183,184]. The t1/2s of the relatively unstable E or Z isomers, which changed depending on the chemical structure, were up to ten thousand years at 25 °C in solution and in the bulk [185], although the thermal isomerization strongly depended on the polarity of the media. Photoinduced crystal–isotropic transitions of a hydrazone small molecule were discovered in 2018 [186]. The crystalline Z isomer of a hydrazone derivative with a dimethyl amino group changed to isotropic with fluorescence quenching upon blue light irradiation because the E isomer was noncrystalline and nonfluorescent (Fig. 4d, e). The crystal and fluorescence were partially restored by UV exposure. Similarly, photoinduced crystal–liquid transitions were observed in macrocyclic hydrazones (X = C8 and C9 in Fig. 4f) and used for energy storage and release [185]. Although such transitions did not occur at room temperature, photoinduced Tm changes (ΔTm < 60 °C) were possible with other acyclic and cyclic hydrazone molecules (Fig. 4f, g) [185, 187]. Interestingly, the E isomers of the macrocycles with short alkyl linkers (X = C6O1 and C7) had higher Tms than the corresponding Z isomers, which indicates that the opposite direction, i.e., transitions between liquid Z isomers and solid E isomers, will be also possible in hydrazone small molecules by optimizing the modification.
Hydrazone polymers
Linear poly(meth)acrylate homopolymers of hydrazone changed their Tgs upon light irradiation (Fig. 4h) [188]. Purple light irradiation of the stable Z isomers produced the metastable E isomers and increased the Tgs by up to 22 °C. The effects of the side chain spacer (m) and tail (n) lengths, substitution position (ortho, meta, or para), and main chain backbone (acrylate or methacrylate) were investigated. Finally, the Tgs of both isomers were tuned to around room temperature (20–37 °C) with ΔTg of 15 or 16 °C (para, R = H, m = 2, n = 9 or 11).
Diarylethene small molecule
Isomerization between the thermally stable open and closed forms of DAE proceeds via UV and visible irradiation, and the conjugation changes have attracted considerable interest (Fig. 1) [189, 190]. Although the small structural changes are considered unsuitable, photoinduced crystal-to-liquid transitions were found for a DAE derivative (Fig. 4i) [191]. The open-ring and closed-ring isomers were crystalline at room temperature with Tms of ca. 100 °C and ca. 140 °C, respectively, while the Tm of their mixture decreased to approximately 30 °C. UV light exposure of the open-ring crystals generated the closed-ring isomer and caused liquefaction of the surface due to the lowered Tm of the mixture. Microfibrils of the closed-ring isomer formed on the surface over 24 h in the dark and disappeared during visible light irradiation.
1,2-Diketone small molecule
An asymmetric heteroaromatic 1,2-diketone consisting of thiophene and furan rings exhibited room-temperature phosphorescence (RTP) in the crystalline and supercooled liquid states (Fig. 4j) [192]. The diketone mainly exists in the stable skew and metastable planar conformations. While the skew conformer forms the crystals and produces feeble green RTP, the planar conformer is more stable in the excited state and yields strong yellow RTP in the liquid state. Very recently, the diketone was reported to show a photoinduced crystal-to-liquid transition at room temperature with RTP changes [193]. UV irradiation macroscopically melted the crystals into a liquid after disappearance of the initial green RTP and emergence of the yellow RTP (Fig. 4j). The mechanism was elucidated with real-time monitoring of the RTP changes, single crystal X-ray structural analyzes at different temperatures and after UV light irradiation, and density functional theory calculations with comparisons to the corresponding symmetric heteroaromatic 1,2-diketones [194,195,196]. A symmetric diketone composed of two furan rings also changed from crystals to a liquid without luminescence upon UV exposure. Although recrystallization from the liquid asymmetric diketone was extremely slow and required several months [192], spontaneous and quick liquid-to-crystal transitions after photoinduced melting and on-demand liquid-to-crystal transitions caused by an external stimulus will be possible after modification of the chemical structure based on the elucidated mechanism. Additionally, photoinduced transitions of polymers with diketones will be fascinating [197].
Conclusion
In this article, we reviewed the recent progress made with photoinduced transitions of small molecules and polymers between the solid, glass, and liquid states based on molecular photoswitches. Only a few photoswitches were found to undergo photoinduced transitions, and those based on AB have been intensively studied for approximately a decade. Therefore, the mechanisms and structural requirements for the transitions are poorly understood. Additionally, AB is unsuitable for some applications due to the thermal instability and high polarity of the Z isomer. Photoinduced transitions should be studied with a wide variety of photoswitches. In particular, thermally stable photoswitches such as hydrazone [179,180,181,182,183,184,185,186,187,188], DAE [189,190,191], hemiindigo (HI) [198], hemithioindigo (HTI) [198, 199], stiff stilbene (SS) [200], and sterically hindered SS (HSS) [201, 202] (Fig. 5) and visible and NIR light-responsive switches [203, 204] are promising. HI and HTI combine both thermal stability and two-way isomerization induced by visible light. SS and HSS have already been reported to show largely different Tms in the E and Z isomers [203]. We believe that future studies on these photoinduced transitions will completely elucidate the mechanisms, establish design guidelines, broaden the use, and accelerate practical applications.
References
Yamamoto T, Norikane Y, Akiyama H. Photochemical liquefaction and softening in molecular materials, polymers, and related compounds. Polym J 2018;50:551–62.
Weis P, Tian W, Wu S. Photoinduced liquefaction of azobenzene-containing polymers. Chem Eur J 2018;24:6494–505.
Xu W, Sun S, Wu S. Photoinduced reversible solid-to-liquid transitions for photoswitchable. Mater Angew Chem Int Ed 2019;58:9712–40.
Hu J, Song T, Yu MM, Yu H. Optically controlled solid-to-liquid phase transition materials based on azo compounds. Chem Mater 2023;35:4621–48.
Hohl DK, Weder C. (De)bonding on demand with optically switchable adhesives. Adv Opt Mater. 2019;7:1900230
Xu G, Li S, Liu C, Wu S. Photoswitchable adhesives using azobenzene-containing materials. Chem Asian J 2020;15:547–54.
Dong L, Feng Y, Wang L, Feng W. Azobenzene-based solar thermal fuels: design, properties, and applications. Chem Soc Rev 2018;47:7339–68.
Gerkman MA, Han GGD. Toward controlled thermal energy storage and release in organic phase change materials. Joule. 2020;4:1621–5.
Wang Z, Erhart P, Li T, Zhang ZY, Sampedro D, Hu Z, et al. Storing energy with molecular photoisomers. Joule. 2021;5:3116–36.
Wu S, Li T, Zhang ZY, Li T, Wang R. Photoswitchable phase change materials for unconventional thermal energy storage and upgrade. Matter. 2021;4:3385–99.
Xu X, Wang G. Molecular solar thermal systems towards phase change and visible light photon energy storage. Small. 2022;18:2107473.
Le M, Han GGD. Stimuli-responsive organic phase change materials: molecular designs and applications in energy storage. Acc Mater Res. 2022;3:634–43.
Li X, Cho S, Wan J, Han GGD. Photoswitches and photochemical reactions for optically controlled phase transition and energy storage. Chem. 2023;9:2378–89.
Yang B, Yu M, Yu H. Azopolymer-based nanoimprint lithography: recent developments in methodology and applications. ChemPlusChem. 2020;85:2166–76.
Lancia F, Ryabchun A, Katsonis N. Life-like motion driven by artificial molecular machines. Nat Rev Chem 2019;3:536–51.
Kamperman M, Synytska A. Switchable adhesion by chemical functionality and topography. J Mater Chem 2012;22:19390–401.
Croll AB, Hosseini N, Bartlett MD. Switchable adhesives for multifunctional interfaces. Adv Mater Technol 2019;4:1900193.
Blelloch ND, Yarbrough HJ, Mirica KA. Stimuli-responsive temporary adhesives: enabling debonding on demand through strategic molecular design. Chem Sci 2021;12:15183–205.
Mulcahy KR, Kilpatrick AFR, Harper GDJ, Walton A, Abbott AP. Debondable adhesives and their use in recycling. Green Chem. 2021;24:36–61.
Liu Z, Yan F. Switchable adhesion: on-demand bonding and debonding. Adv Sci 2022;9:2200264.
Tannouri P, Arafeh KM, Krahn JM, Beaupré SL, Menon C, Branda NR. A photoresponsive biomimetic dry adhesive based on doped PDMS microstructures. Chem Mater 2014;26:4330–3.
Mostafavi SH, Tong F, Dugger TW, Kisailus D, Bardeen CJ. Noncovalent photochromic polymer adhesion. Macromolecules. 2018;51:2388–94.
Mostafavi SH, Li W, Clark KD, Stricker F, Alaniz JRde, Bardeen CJ. Photoinduced deadhesion of a polymer film using a photochromic donor–acceptor Stenhouse adduct. Macromolecules. 2019;52:6311–7.
Gately TJ, Li W, Mostafavi SH, Bardeen CJ. Reversible adhesion switching using spiropyran photoisomerization in a high glass transition temperature polymer. Macromolecules. 2021;54:9319–26.
Imato K, Momota K, Kaneda N, Imae I, Ooyama Y. Photoswitchable adhesives of spiropyran polymers. Chem Mater 2022;34:8289–96.
Zhang L, Deng Y, Xie C, Wu Z. Disordered low molecular weight spiropyran exhibiting photoregulated adhesion ability. Chem Eur J 2022;28:e202200245.
Trenor SR, Long TE, Love BJ. Development of a light-deactivatable PSA via photodimerization. J Adhes 2005;81:213–29.
Asadirad AM, Boutault S, Erno Z, Branda NR. Controlling a polymer adhesive using light and a molecular switch. J Am Chem Soc. 2014;136:3024–7.
Harper T, Slegeris R, Pramudya I, Chung H. Single-phase photo-cross-linkable bioinspired adhesive for precise control of adhesion strength. ACS Appl Mater Interfaces. 2017;9:1830–9.
Kaiser S, Radl S V, Manhart J, Ayalur-Karunakaran S, Griesser T, Moser A, et al. Switching “on” and “off” the adhesion in stimuli-responsive elastomers. Soft Matter. 2018;14:2547–59.
Akiyama H, Okuyama Y, Fukata T, Kihara H. Reversible photocuring of liquid hexa-anthracene compounds for adhesive applications. J Adhes 2018;94:799–813.
Zhou Y, Zhang C, Gao S, Li W, Kai J, Wang Z. Pressure-sensitive adhesive with enhanced and phototunable underwater adhesion. ACS Appl Mater Interfaces. 2021;13:50451–60.
Oka M, Takagi H, Miyazawa T, Waymouth RM, Honda S. Photocleavable regenerative network materials with exceptional and repeatable viscoelastic manipulability. Adv Sci 2021;8:2101143.
Aizawa M, Akiyama H, Matsuzawa Y. Dismantlable adhesion interface featuring a thermo/photocleavable molecular layer. Adv Eng Mater 2022;24:2100823.
Aizawa M, Akiyama H, Yamamoto T, Matsuzawa Y. Photo-and heat-induced dismantlable adhesion interfaces prepared by layer-by-layer deposition. Langmuir. 2023;39:2771–8.
Inada M, Horii T, Fujie T, Nakanishi T, Asahi T, Saito K. Debonding-on-demand adhesives based on photo-reversible cycloaddition reactions. Mater Adv 2023;4:1289–96.
Saito S, Nobusue S, Tsuzaka E, Yuan C, Mori C, Hara M, et al. Light-melt adhesive based on dynamic carbon frameworks in a columnar liquid-crystal phase. Nat Commun 2016;7:12094.
Pianowski ZL. Recent Implementations of molecular photoswitches into smart materials and biological systems. Chem Eur J 2019;25:5128–44.
Boelke J, Hecht S. Designing molecular photoswitches for soft materials applications. Adv Opt Mater 2019;7:1900404.
Dattler D, Fuks G, Heiser J, Moulin E, Perrot A, Yao X, et al. Design of collective motions from synthetic molecular switches, rotors, and motors. Chem Rev 2020;120:310–433.
Volarić J, Szymanski W, Simeth NA, Feringa BL. Molecular photoswitches in aqueous environments. Chem Soc Rev 2021;50:12377–449.
Lu P, Ahn D, Yunis R, Delafresnaye L, Corrigan N, Boyer C, et al. Wavelength-selective light-matter interactions in polymer science. Matter. 2021;4:2172–229.
Norikane Y, Hirai Y, Yoshida M. Photoinduced isothermal phase transitions of liquid-crystalline macrocyclic azobenzenes. Chem Commun 2011;47:1770–2.
Ikeda T. Photomodulation of liquid crystal orientations for photonic applications. J Mater Chem 2003;13:2037–57.
Yu H, Ikeda T. Photocontrollable liquid-crystalline actuators. Adv Mater 2011;23:2149–80.
Yu H. Recent advances in photoresponsive liquid-crystalline polymers containing azobenzene chromophores. J Mater Chem C. 2014;2:3047–54.
Bisoyi HK, Li Q. Light-directed dynamic chirality inversion in functional self-organized helical superstructures. Angew Chem Int Ed 2016;55:2994–3010.
Bisoyi HK, Li Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications. Chem Rev 2016;116:15089–166.
Wang T, Li X, Dong Z, Huang S, Yu H. Vertical orientation of nanocylinders in liquid-crystalline block copolymers directed by light. ACS Appl Mater Interfaces. 2017;9:24864–72.
Huang S, Chen Y, Ma S, Yu H. Hierarchical self-assembly in liquid-crystalline block copolymers enabled by chirality transfer. Angew Chem Int Ed. 2018;57:12524–8.
Pang X, Lv J, Zhu C, Qin L, Yu Y. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv Mater 2019;31:1904224.
Ji Y, Yang B, Cai F, Yu H. Regulating surface topography of liquid-crystalline polymers by external stimuli. Macromol Chem Phys 2022;223:2100418.
Cai F, Yang B, Yu M, Zeng S, Yu H. Photocontrollable liquid-crystalline block copolymers: design, photo-directed self-assembly and applications. J Mater Chem C. 2023;11:3180–96.
Yang B, Ji Y, Cai F, Yu H. Surface morphing of azopolymers toward advanced anticounterfeiting enabled by a two-step method: light writing and then reading in liquid. ACS Appl Mater Interfaces. 2023;15:23804–12.
Bandara HMD, Burdette SC. Photoisomerization in different classes of azobenzene. Chem Soc Rev 2012;41:1809–25.
Gao M, Kwaria D, Norikane Y, Yue Y. Visible-light-switchable azobenzenes: Molecular design, supramolecular systems, and applications. Nat Sci 2023;3:e220020.
Eligehausen S, Sarge SM, Öhlschläger G, Cammenga HK. The pseudobinary phase diagram cis-/trans-azobenzene and the cis → trans isomerization in various states of aggregation. J Therm Anal 1989;35:515–26.
Bhattacharjee U, Freppon D, Men L, Vela J, Smith EA, Petrich JW. Photoinduced trans-to-cis phase transition of polycrystalline azobenzene at low irradiance occurs in the solid state. ChemPhysChem. 2017;18:2526–32.
Uchida E, Sakaki K, Nakamura Y, Azumi R, Hirai Y, Akiyama H, et al. Control of the orientation and photoinduced phase transitions of macrocyclic azobenzene. Chem Eur J 2013;19:17391–7.
Hoshino M, Uchida E, Norikane Y, Azumi R, Nozawa S, Tomita A, et al. Crystal melting by light: x-ray crystal structure analysis of an azo crystal showing photoinduced crystal-melt transition. J Am Chem Soc 2014;136:9158–64.
Okui Y, Han M. Rational design of light-directed dynamic spheres. Chem Commun 2012;48:11763–5.
Akiyama H, Yoshida M. Photochemically reversible liquefaction and solidification of single compounds based on a sugar alcohol scaffold with multi azo-arms. Adv Mater 2012;24:2353–6.
Akiyama H, Yoshida M, Kihara H, Norikane Y, Azumi R. Organic photofunctional materials composed of azobenzene derivatives: liquid-solid phase transition in multi azobenzene compounds with partially substituted. Struct J Photopolym Sci Technol 2014;27:301–5.
Akiyama H, Kanazawa S, Yoshida M, Kihara H, Nagai H, Norikane Y, et al. Photochemical liquid–solid transitions in multi-dye compounds. Mol Cryst Liq Cryst 2014;604:64–70.
Akiyama H, Kanazawa S, Okuyama Y, Yoshida M, Kihara H, Nagai H, et al. Photochemically reversible liquefaction and solidification of multiazobenzene sugar-alcohol derivatives and application to reworkable adhesives. ACS Appl Mater Interfaces. 2014;6:7933–41.
Norikane Y, Uchida E, Tanaka S, Fujiwara K, Koyama E, Azumi R, et al. Photoinduced crystal-to-liquid phase transitions of azobenzene derivatives and their application in photolithography processes through a solid–liquid patterning. Org Lett 2014;16:5012–5.
Norikane Y, Uchida E, Tanaka S, Fujiwara K, Nagai H, Akiyama H. Photoinduced phase transitions in rod-shaped azobenzene with different alkyl chain length. J Photopolym Sci Technol 2016;29:149–57.
Kikkawa Y, Tanaka S, Norikane Y. Photo-triggered enzymatic degradation of biodegradable polymers. RSC Adv. 2017;7:55720–4.
Hu J, Li X, Ni Y, Ma S, Yu H. A programmable and biomimetic photo-actuator: a composite of a photo-liquefiable azobenzene derivative and commercial plastic film. J Mater Chem C. 2018;6:10815–21.
Fan G, Wang S, Jiang J, Liu Z, Liu Z, Li G. Rubber-like composites with tunable thermal- and photo-responsive shape memory properties. Chem Eng J 2022;447:137534.
Kim DY, Lee SA, Kim H, Kim SM, Kim N, Jeong KU. An azobenzene-based photochromic liquid crystalline amphiphile for a remote-controllable light shutter. Chem Commun 2015;51:11080–3.
Ishiba K, Morikawa M, Chikara C, Yamada T, Iwase K, Kawakita M, et al. Photoliquefiable ionic crystals: a phase crossover approach for photon energy storage materials with functional multiplicity. Angew Chem Int Ed 2015;54:1532–6.
Nobuoka K, Kitaoka S, Yamauchi T, Harran T, Ishikawa Y. Photoresponsive ionic liquids with an azobenzene moiety. Chem Lett 2016;45:433–5.
Liu H, Zhang L, Zhang G, Du Q, Wang K, Luo X, et al. Molecular design of azobenzene-containing photoresponsive phase change materials with energy storage and photolithography potentials. Dyes Pigment. 2023;215:111300.
Baroncini M, d’Agostino S, Bergamini G, Ceroni P, Comotti A, Sozzani P, et al. Photoinduced reversible switching of porosity in molecular crystals based on star-shaped azobenzene tetramers. Nat Chem 2015;7:634–40.
Li X, Cho S, Han GGD. Light-responsive solid–solid phase change materials for photon and thermal energy storage. ACS Mater Au. 2023;3:37–42.
Uchida E, Azumi R, Norikane Y. Light-induced crawling of crystals on a glass surface. Nat Commun 2015;6:7310.
Norikane Y, Tanaka S, Uchida E. Azobenzene crystals swim on water surface triggered by light. CrystEngComm. 2016;18:7225–8.
Saito K, Ohnuma M, Norikane Y. Negative phototactic behaviour of crystals on a glass surface. Chem Commun 2019;55:9303–6.
Hu J, Huang S, Yu M, Yu H. Flexible solar thermal fuel devices: composites of fabric and a photoliquefiable azobenzene derivative. Adv Energy Mater 2019;9:1901363.
Norikane Y, Hayashino M, Ohnuma M, Abe K, Kikkawa Y, Saito K, et al. Effect of surface properties on the photo-induced crawling motion of azobenzene crystals on glass surfaces. Front Chem 2021;9:684767.
Qiu Q, Gerkman MA, Shi Y, Han GGD. Design of phase-transition molecular solar thermal energy storage compounds: compact molecules with high energy densities. Chem Commun 2021;57:9458–61.
Dong L, Zhai F, Wang H, Peng C, Feng Y, Feng W. An azobenzene-based photothermal energy storage system for co-harvesting photon energy and low-grade ambient heat via a photoinduced crystal-to-liquid transition. Energy Mater. 2022;2:200025.
Tang J, Feng Y, Feng W. Photothermal storage and controllable release of a phase-change azobenzene/aluminum nitride aerogel composite. Compos Commun 2021;23:100575.
Migulin D, Vysochinskaya Y, Buzin M, Bakirov A, Cherkaev G, Shchegolikhina O. Stereoregular hybrid azobenzene-cyclosiloxanes with photoinduced reversible solid to liquid transition properties. J Photochem Photobiol A. 2021;407:113033.
Yang Y, Huang S, Ma Y, Yi J, Jiang Y, Chang X, et al. Liquid and photoliquefiable azobenzene derivatives for solvent-free molecular solar thermal fuels. ACS Appl Mater Interfaces. 2022;14:35623–34.
Yang Q, Ge J, Qin M, Wang H, Yang X, Zhou X, et al. Controllable heat release of phase-change azobenzenes by optimizing molecular structures for low-temperature energy utilization. Sci China Mater 2023;66:3609–20.
Sadovski O, Beharry AA, Zhang F, Woolley GA. Spectral tuning of azobenzene photoswitches for biological applications. Angew Chem Int Ed 2009;48:1484–6.
Siewertsen R, Neumann H, Buchheim-Stehn B, Herges R, Näther C, Renth F, et al. Highly efficient reversible Z−E photoisomerization of a bridged azobenzene with visible light through resolved S1(nπ*) absorption bands. J Am Chem Soc 2009;131:15594–5.
Beharry AA, Sadovski O, Woolley GA. Azobenzene photoswitching without ultraviolet light. J Am Chem Soc 2011;133:19684–7.
Yang Y, Hughes RP, Aprahamian I. Visible Light switching of a BF2-coordinated azo compound. J Am Chem Soc 2012;134:15221–4.
Bléger D, Schwarz J, Brouwer AM, Hecht S. o-fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J Am Chem Soc 2012;134:20597–600.
Samanta S, McCormick TM, Schmidt SK, Seferos DS, Woolley GA. Robust visible light photoswitching with ortho-thiol substituted azobenzenes. Chem Commun 2013;49:10314–6.
Samanta S, Babalhavaeji A, Dong M, Woolley GA. Photoswitching of ortho-substituted azonium ions by red light in whole blood. Angew Chem Int Ed 2013;52:14127–30.
Samanta S, Beharry AA, Sadovski O, McCormick TM, Babalhavaeji A, Tropepe V, et al. Photoswitching azo compounds in vivo with red light. J Am Chem Soc 2013;135:9777–84.
Yang Y, Hughes RP, Aprahamian I. Near-infrared light activated azo-BF2 switches. J Am Chem Soc 2014;136:13190–3.
Hammerich M, Schütt C, Stähler C, Lentes P, Röhricht F, Höppner R, et al. Heterodiazocines: synthesis and photochromic properties, trans to cis switching within the bio-optical. Window J Am Chem Soc 2016;138:13111–4.
Qian H, Wang YY, Guo DS, Aprahamian I. Controlling the isomerization rate of an Azo-BF2 switch using. Aggreg J Am Chem Soc 2017;139:1037–40.
Dong M, Babalhavaeji A, Collins CV, Jarrah K, Sadovski O, Dai Q, et al. Near-infrared photoswitching of azobenzenes under physiological conditions. J Am Chem Soc 2017;139:13483–6.
Ahmed Z, Siiskonen A, Virkki M, Priimagi A. Controlling azobenzene photoswitching through combined ortho fluorination and -amination. Chem Commun 2017;53:12520–3.
Lentes P, Stadler E, Röhricht F, Brahms A, Gröbner J, Sönnichsen FD, et al. Nitrogen bridged diazocines: photochromes switching within the near-infrared region with high quantum yields in organic solvents and in water. J Am Chem Soc 2019;141:13592–13600.
Konrad DB, Savasci G, Allmendinger L, Trauner D, Ochsenfeld C, Ali AM. Computational design and synthesis of a deeply red-shifted and bistable azobenzene. J Am Chem Soc 2020;142:6538–47.
Moormann W, Tellkamp T, Stadler E, Röhricht F, Näther C, Puttreddy R, et al. Efficient conversion of light to chemical energy: directional, chiral photoswitches with very high quantum yields. Angew Chem Int Ed 2020;59:15081–6.
Weston CE, Richardson RD, Haycock PR, White AJP, Fuchter MJ. Arylazopyrazoles: azoheteroarene photoswitches offering quantitative isomerization and long thermal half-lives. J Am Chem Soc 2014;136:11878–81.
Calbo J, Weston CE, White AJP, Rzepa HS, Contreras-García J, Fuchter MJ. Tuning azoheteroarene photoswitch performance through heteroaryl design. J Am Chem Soc 2017;139:1261–74.
Kumar P, Srivastava A, Sah C, Devi S, Venkataramani S. Arylazo-3,5-dimethylisoxazoles: azoheteroarene photoswitches exhibiting high Z-Isomer stability, solid-state photochromism, and reversible light-induced phase transition. Chem Eur J 2019;25:11924–32.
Calbo J, Thawani AR, Gibson RSL, White AJP, Fuchter MJ. A combinatorial approach to improving the performance of azoarene photoswitches. Beilstein J Org Chem 2019;15:2753–64.
Crespi S, Simeth NA, König B. Heteroaryl azo dyes as molecular photoswitches. Nat Rev Chem 2019;3:133–46.
Fang D, Zhang ZY, Shangguan Z, He Y, Yu C, Li T. (Hetero)arylazo-1,2,3-triazoles: "clicked" photoswitches for versatile functionalization and electronic decoupling. J Am Chem Soc. 2021;143:14502–10.
Dang T, Dong D, Zhang J, He Y, Zhang Z, Li T. Thiazolylazopyrazoles as nonsymmetric bis-heteroaryl azo switches: high-yield visible-light photoisomerization and increased Z-isomer stability by o-carbonylation. Angew Chem Int Ed 2023;62:e202301992.
Lin R, Hashim PK, Sahu S, Amrutha AS, Cheruthu NM, Thazhathethil S, et al. Phenylazothiazoles as visible-light photoswitches. J Am Chem Soc 2023;145:9072–80.
Mukherjee A, Seyfried MD, Ravoo BJ. Azoheteroarene and diazocine molecular photoswitches: self-assembly, responsive materials and photopharmacology. Angew Chem Int Ed 2023;62:e202304437.
Shi Y, Gerkman MA, Qiu Q, Zhang S, Han GGD. Sunlight-activated phase change materials for controlled heat storage and triggered release. J Mater Chem A. 2021;9:9798–808.
Kwaria D, McGehee K, Liu S, Kikkawa Y, Ito S, Norikane Y. Visible-light-photomeltable azobenzenes as solar thermal fuels. ACS Appl Opt Mater 2023;1:633–9.
Kortekaas L, Simke J, Kurka DW, Ravoo BJ. Rapid photoswitching of low molecular weight arylazoisoxazole adhesives. ACS Appl Mater Interfaces. 2020;12:32054–60.
Zhang ZY, He Y, Wang Z, Xu J, Xie M, Tao P, et al. Photochemical phase transitions enable coharvesting of photon energy and ambient heat for energetic molecular solar thermal batteries that upgrade thermal energy. J Am Chem Soc 2020;142:12256–64.
Gerkman MA, Gibson RSL, Calbo J, Shi Y, Fuchter MJ, Han GGD. Arylazopyrazoles for long-term thermal energy storage and optically triggered heat release below 0 °C. J Am Chem Soc 2020;142:8688–95.
Gonzalez A, Odaybat M, Le M, Greenfield JL, White AJP, Li X, et al. Photocontrolled energy storage in azobispyrazoles with exceptionally large light penetration depths. J Am Chem Soc 2022;144:19430–6.
Meteling HJ, Bosse F, Schlichter L, Tyler BJ, Arlinghaus HF, Ravoo BJ. Versatile surface patterning with low molecular weight photoswitches. Small. 2022;18:2203245.
Huang X, Shangguan Z, Zhang ZY, Yu C, He Y, Fang D, et al. Visible-light-induced reversible photochemical crystal–liquid transitions of azo-switches for smart and robust adhesives. Chem Mater 2022;34:2636–44.
Wu S, Zhang ZY, Li T, Wang R, Li T. Optically-controlled variable-temperature storage and upgrade of thermal energy by photoswitchable phase change materials. ACS Mater Lett 2023;5:2019–27.
Rossi R, Salvini A, Pizzo B, Frediani M, Wiersma DS, Parmeggiani C, et al. Photoresponsive adhesives based on arylazoisoxazoles-containing polymers. Macromol Mater Eng 2023;308:2200504.
Tsuda M, Kuratani K. Isomerization of cis -azobenzene in the solid phase. Bull Chem Soc 1964;37:1284–8.
Han GGD, Li H, Grossman JC. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat Commun 2017;8:1446.
Han GGD, Deru JH, Cho EN, Grossman JC. Optically-regulated thermal energy storage in diverse organic phase-change materials. Chem Commun 2018;54:10722–5.
Fei L, Yin Y, Zhang J, Wang C. A visible energy management by photochromic solar thermal fuel using a color display. Sol RRL. 2020;4:2000499.
Liu H, Tang J, Dong L, Wang H, Xu T, Gao W, et al. Optically triggered synchronous heat release of phase-change enthalpy and photo-thermal energy in phase-change materials at low temperatures. Adv Funct Mater 2021;31:2008496.
Wang Y, Wang S, Shi J, Chen Z, Sheng L, Zhang T. Molecular dynamics study of optically controlled phase change. Mater J Phys Chem C. 2022;126:5443–56.
Song T, Lei H, Cai F, Kang Y, Yu H, Zhang L. Supramolecular cation−π interaction enhances molecular solar thermal fuel. ACS Appl Mater Interfaces. 2022;14:1940–9.
Wang Y, Shi J, Sheng L, Chen Z. Study on the applicability of photoswitch molecules to optically-controlled thermal energy in different organic phase change materials. Chem Eng J 2023;456:141051.
Griffiths K, Halcovitch NR, Griffin JM. Crystalline azobenzene composites as photochemical phase-change materials. N J Chem 2022;46:4057–61.
Zhou H, Xue C, Weis P, Suzuki Y, Huang S, Koynov K, et al. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat Chem 2017;9:145–51.
Akiyama H, Fukata T, Yamashita A, Yoshida M, Kihara H. Reworkable adhesives composed of photoresponsive azobenzene polymer for glass substrates. J Adhes 2017;93:823–30.
Chen M, Yao B, Kappl M, Liu S, Yuan J, Berger R, et al. Entangled azobenzene-containing polymers with photoinduced reversible solid-to-liquid transitions for healable and reprocessable photoactuators. Adv Funct Mater 2020;30:1906752.
Weis P, Hess A, Kircher G, Huang S, Auernhammer GK, Koynov K, et al. Effects of spacers on photoinduced reversible solid-to-liquid transitions of azobenzene-containing polymers. Chem Eur J 2019;25:10946–53.
Zhang Z, Chen M, Schneider I, Liu Y, Liang S, Sun S, et al. Long alkyl side chains simultaneously improve mechanical robustness and healing ability of a photoswitchable polymer. Macromolecules. 2020;53:8562–9.
Ito S, Yamashita A, Akiyama H, Kihara H, Yoshida M. Azobenzene-based (meth)acrylates: controlled radical polymerization, photoresponsive solid–liquid phase transition behavior, and application to reworkable adhesives. Macromolecules. 2018;51:3243–53.
Liang S, Li S, Yuan C, Zhang D, Chen J, Wu S. Polyacrylate backbone promotes photoinduced reversible solid-to-liquid transitions of azobenzene-containing polymers. Macromolecules. 2023;56:448–56.
Zhou Y, Chen M, Ban Q, Zhang Z, Shuang S, Koynov K, et al. Light-switchable polymer adhesive based on photoinduced reversible solid-to-liquid transitions. ACS Macro Lett. 2019;8:968–72.
Shin J, Sung J, Kang M, Xie X, Lee B, Lee KM, et al. Light-triggered thermal conductivity switching in azobenzene polymers. Proc Natl Acad Sci USA. 2019;116:201817082.
Zhang Z, He Y, Zhou Y, Yu C, Han L, Li T. Pyrazolylazophenyl ether-based photoswitches: facile synthesis, (near-)quantitative photoconversion, long thermal half-life, easy functionalization, and versatile applications in light-responsive systems. Chem Eur J 2019;25:13402–10.
Lin KT, Chen YJ, Huang MR, Karapala VK, Ho JH, Chen JT. Light-induced nanowetting: erasable and rewritable polymer nanoarrays via solid-to-liquid transitions. Nano Lett. 2020;20:5853–9.
Xu J, Niu B, Guo S, Zhao X, Li X, Peng J, et al. Influence of chromophoric electron-donating groups on photoinduced solid-to-liquid transitions of azopolymers. Polymers. 2020;12:901.
Yang B, Cai F, Huang S, Yu H. Athermal and soft multi-nanopatterning of azopolymers: phototunable mechanical properties. Angew Chem Int Ed 2020;59:4035–42.
Pessoni L, Siniscalco D, Boussonnière A, Castanet AS, Billon L, Delorme N. Photo-reversible solid to liquid transition of azobenzene containing polymers: Impact of the chemical structure and chain length. Eur Polym J 2022;174:111297.
Shang C, Xiong Z, Liu S, Yu W. Molecular dynamics of azobenzene polymer with photoreversible glass transition. Macromolecules. 2022;55:3711–22.
Wan L, Min X, Yue W, Lin F, Feng J, Liu X, et al. Polymer with variable thermal conductivity regulated by photoirradiation. ACS Appl Polym Mater 2023;5:6540–8.
Ito S, Akiyama H, Mori M, Yoshida M, Kihara H. Semicrystalline poly(vinyl ether)s with high and phototunable glass transition temperature: application for thermally stable and reworkable adhesives. J Polym Sci 2020;58:568–77.
Xu W, Liu C, Liang S, Zhang D, Liu Y, Wu S. Designing rewritable dual-mode patterns using a stretchable photoresponsive polymer via orthogonal photopatterning. Adv Mater 2022;34:e2202150.
Zhao R, Mu J, Bai J, Zhao W, Gong P, Chen L, et al. Smart responsive azo-copolymer with photoliquefaction for switchable adhesive application. ACS Appl Mater Interfaces. 2022;14:16678–86.
Tol JJB, Engels TAP, Cardinaels R, Vantomme G, Meijer EW, Eisenreich F. Photoswitchable liquid-to-solid transition of azobenzene-decorated polysiloxanes. Adv Funct Mater 2023;33:2301246.
Ito S, Akiyama H, Sekizawa R, Mori M, Yoshida M, Kihara H. Light-induced reworkable adhesives based on ABA-type triblock copolymers with azopolymer termini. ACS Appl Mater Interfaces. 2018;10:32649–58.
Ito S, Akiyama H, Sekizawa R, Mori M, Fukata T, Yoshida M, et al. Azobenzene-containing block copolymers as light-induced reworkable adhesives: effects of molecular weight, composition, and block copolymer architectures on the adhesive properties. J Polym Sci A. 2019;57:806–13.
Ito S, Akiyama H, Mori M, Yoshida M, Kihara H. Azobenzene-containing triblock copolymer adhesive based on light-induced solid–liquid phase transition: application to bonding for various substrates. Macromol Chem Phys 2019;220:1900105.
Cai F, Yang B, Lv X, Feng W, Yu H. Mechanically mutable polymer enabled by light. Sci Adv 2022;8:eabo1626.
Appiah C, Woltersdorf G, Pérez-Camargo RA, Müller AJ, Binder WH. Crystallization behavior of precision polymers containing azobenzene defects. Eur Polym J 2017;97:299–307.
Li S, Han G, Zhang W. Concise synthesis of photoresponsive polyureas containing bridged azobenzenes as visible-light-driven actuators and reversible photopatterning. Macromolecules. 2018;51:4290–7.
Kuenstler AS, Clark KD, Alaniz JRde, Hayward RC. Reversible actuation via photoisomerization-induced melting of a semicrystalline poly(azobenzene). ACS Macro Lett. 2020;9:902–9.
Zhang P, Cai F, Wang W, Wang G, Yu H. Light-switchable adhesion of azobenzene-containing siloxane-based tough adhesive. ACS Appl Polym Mater 2021;3:2325–9.
Shen D, Yao Y, Zhuang Q, Lin S. Mainchain alternating azopolymers with fast photo-induced reversible transition behavior. Macromolecules. 2021;54:10040–8.
Zha RH, Vantomme G, Berrocal JA, Gosens R, Waal B, de, Meskers S, et al. Photoswitchable nanomaterials based on hierarchically organized siloxane oligomers. Adv Funct Mater 2018;28:1703952.
Lee C, Ndaya D, Bosire R, Kim NK, Kasi RM, Osuji CO. Fast photoswitchable order–disorder transitions in liquid-crystalline block co-oligomers. J Am Chem Soc 2022;144:390–9.
Guo Y, Xiao J, Sun Y, Song B, Zhang H, Dong B. Photoswitching of the melting point of a semicrystalline polymer by the azobenzene terminal group for a reversible solid-to-liquid transition. J Mater Chem A. 2021;9:9364–70.
Yue Y, Norikane Y, Azumi R, Koyama E. Light-induced mechanical response in crosslinked liquid-crystalline polymers with photoswitchable glass transition temperatures. Nat Commun 2018;9:3234.
Zhang X, Zhu C, Xu B, Qin L, Wei J, Yu Y. Rapid, localized, and athermal shape memory performance triggered by photoswitchable glass transition temperature. ACS Appl Mater Interfaces. 2019;11:46212–8.
Xu X, Zhang P, Wu B, Xing Y, Shi K, Fang W, et al. Photochromic dendrimers for photoswitched solid-to-liquid transitions and solar thermal fuels. ACS Appl Mater Interfaces. 2020;12:50135–42.
Yamamoto T, Hasegawa R, Kawata Y, Kihara H, Naga N. Photoplasticization effect of an azobenzene-doped liquid crystal depending on phase structures. Chem Lett 2018;47:272–5.
Koike M, Aizawa M, Akamatsu N, Shishido A, Matsuzawa Y, Yamamoto T. Photoplasticization behavior and photoinduced pressure-sensitive adhesion properties of various polymers containing an azobenzene-doped liquid crystal. Bull Chem Soc 2020;93:1588–94.
Koike M, Aizawa M, Minamikawa H, Shishido A, Yamamoto T. Photohardenable pressure-sensitive adhesives using poly(methyl methacrylate) containing liquid crystal plasticizers. ACS Appl Mater Interfaces. 2021;13:39949–56.
Zhang L, Qu Y, Gu J, Tang Z, Wu Z, Luo X. Photoliquefiable DNA-surfactant ionic crystals: anhydrous self-healing biomaterials at room temperature. Acta Biomater. 2021;128:143–9.
Maeda M, Nobukawa S, Inomata K. Photoinduced plasticizing effect of the addition of azobenzene on the glass transition temperature and mechanical properties of polycarbonate. Polym J 2022;54:269–79.
Zhao S, Yamagishi H, Norikane Y, Hayashi S, Yamamoto Y. Optical control of aggregation-induced emission shift by photoisomerizable precipitant in a liquid droplet microresonator. Adv Opt Mater 2023;11:2202134.
Klajn R. Spiropyran-based dynamic materials. Chem Soc Rev 2014;43:148–84.
Kortekaas L, Browne WR. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chem Soc Rev 2019;48:3406–24.
Imato K, Nagata K, Watanabe R, Takeda N. Cell adhesion control by photoinduced LCST shift of PNIPAAm-based brush scaffolds. J Mater Chem B. 2020;8:2393–9.
Uchida E, Azumi R, Norikane Y. Switching between solid and liquid phases of spiropyran by photochromic reaction. Chem Lett 2014;43:1619–21.
Hu W, Sun C, Ren Y, Qin S, Shao Y, Zhang L, et al. Programmable chromism and photoluminescence of spiropyran-based liquid crystalline polymer with tunable glass transition temperature. Angew Chem Int Ed 2021;60:19406–12.
Pruthi V, Akae Y, Théato P. Photoresponsive spiropyran and DEGMA-based copolymers with photo-switchable glass transition temperatures. Macromol Rapid Commun 2023;44:e2300270.
Dijken DJvan, Kovaříček P, Ihrig SP, Hecht S. Acylhydrazones as widely tunable photoswitches. J Am Chem Soc 2015;137:14982–91.
Qian H, Pramanik S, Aprahamian I. Photochromic hydrazone switches with extremely long thermal half-lives. J Am Chem Soc 2017;139:9140–3.
Li Q, Qian H, Shao B, Hughes RP, Aprahamian I. Building strain with large macrocycles and using it to tune the thermal half-lives of hydrazone photochromes. J Am Chem Soc 2018;140:11829–35.
Shao B, Qian H, Li Q, Aprahamian I. Structure property analysis of the solution and solid-state properties of bistable photochromic hydrazones. J Am Chem Soc 2019;141:8364–71.
Shao B, Aprahamian I. Planarization-induced activation wavelength red-shift and thermal half-life acceleration in hydrazone photoswitches. ChemistryOpen. 2020;9:191–4.
Shao B, Aprahamian I. Hydrazones as new molecular tools. Chem. 2020;6:2162–73.
Qiu Q, Yang S, Gerkman MA, Fu H, Aprahamian I, Han GGD. Photon energy storage in strained cyclic hydrazones: emerging molecular solar thermal energy storage compounds. J Am Chem Soc 2022;144:12627–31.
Shao B, Baroncini M, Qian H, Bussotti L, Donato MD, Credi A, et al. Solution and solid-state emission toggling of a photochromic hydrazone. J Am Chem Soc 2018;140:12323–7.
Koibuchi R, Omasa K, Yoshikawa I, Houjou H. Photoinduced crystal-to-liquid transition of acylhydrazone-based photoswitching molecules. J Phys Chem Lett 2023;14:8320–6.
Yang S, Harris JD, Lambai A, Jeliazkov LL, Mohanty G, Zeng H, et al. Multistage reversible tg photomodulation and hardening of hydrazone-containing polymers. J Am Chem Soc 2021;143:16348–53.
Irie M, Fukaminato T, Matsuda K, Kobatake S. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem Rev 2014;114:12174–277.
Zhang J, Tian H. The endeavor of diarylethenes: new structures, high performance, and bright future. Adv Opt Mater 2018;6:1701278.
Uchida K, Izumi N, Sukata S, Kojima Y, Nakamura S, Irie M. Photoinduced reversible formation of microfibrils on a photochromic diarylethene microcrystalline surface. Angew Chem Int Ed 2006;45:6470–3.
Komura M, Ogawa T, Tani Y. Room-temperature phosphorescence of a supercooled liquid: kinetic stabilisation by desymmetrisation. Chem Sci 2021;12:14363–8.
Komura M, Sotome H, Miyasaka H, Ogawa T, Tani Y. Photoinduced crystal melting with luminescence evolution based on conformational isomerisation. Chem Sci 2023;14:5302–8.
Tani Y, Terasaki M, Komura M, Ogawa T. Room-temperature phosphorescence-to-phosphorescence mechanochromism of a metal-free organic 1,2-diketone. J Mater Chem C. 2019;7:11926–31.
Tani Y, Komura M, Ogawa T. Mechanoresponsive turn-on phosphorescence by a desymmetrization approach. Chem Commun 2020;56:6810–3.
Takewaki Y, Ogawa T, Tani Y. Modulating room-temperature phosphorescence-to-phosphorescence mechanochromism by halogen exchange. Front Chem 2022;9:812593.
Chen Z, Chen X, Ma D, Mao Z, Zhao J, Chi Z. Synergetic conformational regulations in ground and excited states for realizing stimulus-responsive and wide-tuning room-temperature phosphorescence. J Am Chem Soc 2023;145:16748–59.
Wiedbrauk S, Dube H. Hemithioindigo—an emerging photoswitch. Tetrahedron Lett. 2015;56:4266–74.
Petermayer C, Dube H. Indigoid photoswitches: visible light responsive molecular tools. Acc Chem Res 2018;51:1153–63.
Villarón D, Wezenberg SJ. Stiff-stilbene photoswitches: from fundamental studies to emergent applications. Angew Chem Int Ed 2020;59:13192–202.
Imato K, Sasaki A, Ishii A, Hino T, Kaneda N, Ohira K, et al. Sterically hindered stiff-stilbene photoswitch offers large motions, 90% two-way photoisomerization, and high thermal. Stab J Org Chem 2022;87:15762–70.
Imato K, Ishii A, Kaneda N, Hidaka T, Sasaki A, Imae I, et al. Thermally stable photomechanical molecular hinge: sterically hindered stiff-stilbene photoswitch mechanically isomerizes. JACS Au. 2023;3:2458–66.
Zhang Z, Wang W, O’Hagan M, Dai J, Zhang J, Tian H. Stepping out of the blue: from visible to near-IR triggered photoswitches. Angew Chem Int Ed. 2022;61:e202205758.
Leistner A, Pianowski ZL. Smart photochromic materials triggered with visible light. Eur J Org Chem 2022;2022:e202101271.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Nos. 19K15623, 21H01987, and 21H05884), MEXT LEADER (Grant No. A6501), and JST PRESTO (Grant No. JPMJPR21N2).
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imato, K., Kaneda, N. & Ooyama, Y. Recent progress in photoinduced transitions between the solid, glass, and liquid states based on molecular photoswitches. Polym J 56, 269–282 (2024). https://doi.org/10.1038/s41428-023-00873-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00873-7