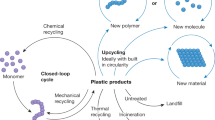
Abstract
Over 8 billion tons of plastic have been produced to date, and a 100% reclamation recycling strategy is not foreseeable. This review summarizes how the mechanochemistry of polymers may contribute to a sustainable polymer future by controlling the degradation not only of de novo developed designer polymers but also of plastics in existing waste streams. The historical development of polymer mechanochemistry is presented while highlighting current examples of mechanochemically induced polymer degradation. Additionally, theoretical and computational frameworks are discussed that may lead to the discovery and better understanding of new mechanochemical reactions in the future. This review takes into account technical and engineering perspectives converging the fields of trituration and polymer mechanochemistry with a particular focus on the fate of commodity polymers and potential technologies to monitor mechanochemical reactions while they occur. Therefore, a unique perspective of multiple communities is presented, highlighting the need for future transdisciplinary research to tackle the high-leverage parameters governing an eventually successful mechanochemical degradation approach for a circular economy.
Similar content being viewed by others
Introduction
The use of mechanochemical reactions for the chemical recycling of polymers is a relatively new concept, although the underlying phenomena have been described for decades. Initially, the widespread introduction of polymers as commodity materials necessitated the investigation of their behavior under mechanical action. In 1934, Staudinger and Heuer observed that the degradation of polystyrene (PS) and natural rubber led to a decreasing viscosity and hence attributed this to a decreasing molar mass. They hypothesized that this was caused by the mechanochemically induced depolymerization of the polymer backbone [1]. Kauzmann and Eyring confirmed this hypothesis in 1940 and developed the first kinetic description of the mechanochemical bond scission process in polymers [2].
The last decade has experienced rapid growth of polymer mechanochemistry employing tailored polymers that bear force-sensitive functional molecular motifs (mechanophores) [3,4,5]. These mechanophores were placed in different polymer architectures, and their mechanochemical actuation resulted in specific molecular transformations realizing the desired function. Although it is a promising technology, the mechanophore concept still suffers from some unsolved limitations, such as expensive syntheses, processability, and alteration of the physicochemical properties of the host polymer matrix, rendering scalability and transfer to application problematic [6,7,8].
An alternative approach to exert force on molecules and materials is the use of trituration mechanochemistry [9], which is defined as the realization of chemical reactions by direct absorption of mechanical energy exerted by grinding and milling [10]. In contrast to polymer mechanochemistry, trituration mechanochemistry relies on the compression and shearing of molecules and is hence mostly associated with bond formation reactions. Trituration mechanochemistry is often conducted in ball mills, where mechanical energy is delivered by collisions. This allows solid‒solid reactions to be performed in a solvent-free environment or with little solvent [11] and enables reactions that normally require high temperature to activate reactants and rapid quenching to prevent undesirable series reactions [12]. In addition, ball mills are an established and scalable technology industrially used in ore and concrete processing with performances of multiple thousand tons per hour.
Very recently, the historically separated fields of polymer and trituration mechanochemistry started to converge, as researchers found that milling and grinding are very much suited to mechanochemically synthesize or degrade polymers – with and without mechanophores [13,14,15]. This has led to a recent rapid increase in reported research where on the one hand, trituration techniques are being used to indiscriminately pulverize and breakdown polymer materials for sustainability applications, and, on the other hand, tailored mechanophores in the polymer mechanochemistry community are selectively activated using milling procedures. While the polymer mechanochemistry community struggles with the aforementioned hurdles for real-world applications, the trituration mechanochemistry community has not yet implemented the full conceptual framework of polymer mechanochemistry that has been established over the last few decades [16]. This is complicated by conceptually unclear obstacles, such as the alignment of the reactive bonds with the force vector. While polymer mechanochemical reactions are mostly dissociative and hence bonds are stretched while the reacting atoms are moved away from each other on the reaction coordinate, the same mechanism cannot be applied to trituration where a mixture of shear and compressive forces occur. In addition to direction, the intensity, duration, and rate of mechanical force application play an elusive role in mechanochemical reactions. This leads, among others, to problems in the theoretical description of force-induced trituration reactions.
This review aims to facilitate this disciplinary exchange and to catalyze future endeavors in the context of sustainable polymer degradation so that both communities can profit from their respective established principles and procedures. Therefore, we summarize recent notable examples and discuss a possible future path with challenges and obstacles to be overcome. We introduce tailor-made polymers adhering to the mechanophore concept that depolymerize partially or completely upon force application. We then summarize the theoretical and computational frameworks that are necessary to describe the mechanochemical reactivity of mechanophores to render them applicable in real-world sustainability scenarios. Hereafter, we provide an overview of trituration mechanochemistry efforts in combination with heterogeneous catalysis and their potential in depolymerization reactions. Last, we highlight current in situ mechanochemical observation techniques to tackle the big challenge of how to rationalize mechanochemistry in ball milling processes.
Mechanophores for the accelerated degradation of polymers
A fundamental requirement for a degradable polymer is that the stimulus initiating the degradation reaction must be orthogonal to the application profile of the polymer. For example, it would be disadvantageous to endow a polymer used for organic photovoltaics with light-induced degradability. One approach to circumvent this problem is to gate the degradation stimulus by an additional functionality. Particularly in the field of mechanochemistry, this approach is useful since mechanical force is ubiquitous and acts on polymers during their manufacturing and application [15]. To construct a molecular gate, a secondary gated reactivity is masked by a primary gating function. Force is currently reported exclusively as a primary gating function, while secondary reactivity can be either an increased susceptibility to degradation reagents (acids, bases, catalysts) or a self-immolative function [17, 18]. These efforts complement research activities on mechanochemical bond scission reinforcement reactions that can be used to prolong the material life cycle through inducing secondary polymerizations [19,20,21,22,23] or the dissipation of mechanical energy by mechanophores as sacrificial bonds [24,25,26].
Ceiling temperature engineering
In 2014, Moore and coworkers presented what could be described as the first mechanochemically degradable polymer with specifically tailored force-responsive functionality [27]. They prepared cyclic poly(o-phthalaldehyde) (PPA) with a high molar mass and subjected it to ultrasound (US) [28] in solution (Fig. 1a). In addition to the expected chain scission and average molar mass decrease, they observed depolymerization of the polymer. The PA monomer was produced and could be effectively repolymerized into PPA using an anionic initiator, thus accomplishing a full cycle of depolymerization and repolymerization. With combined experimental trapping techniques and ab initio computational simulations, they found that mechanochemical scission proceeded in an unusual (for polymer mechanochemistry) heterolytic fashion, producing both hemiacetalate and oxocarbenium end groups. These reactive sites effectively lowered the ceiling temperature Tc of the polymer, consequently initiating depolymerization even under the given sonication conditions at −15 °C. While US-induced inertial cavitation can create hot spots with very high temperatures [29, 30], this did not cause the depolymerization reaction of the low-Tc PPA, as verified on shorter, mechanochemically inactive PPA derivatives.
Mechanochemically induced depolymerization reactions. The force-responsive bond is depicted in red, and the degradable moiety is depicted in green. a Poly(o-phthalaldehyde) by Moore and coworkers [27]. b Poly(vinyl acetate-alt-sulfur dioxide) by Kumar and Goodwin [31]. c Poly(cyclobutane lactone) by Wang and coworkers [33]. d Poly(cyclobutane ketal) by Craig and coworkers [34]. e Poly(cyclobutene diol) by Craig and coworkers [35]. f Latent poly (2,5-dihydrofuran) by Wang and coworkers [36]. gem-Dichlorocyclopropane (gDCC) mechanophores in Panels (d) and (e) are shown but were only used as internal standards for scission rate analysis
A comparable approach relying on a low-Tc polymer was developed by Kumar and Goodwin in 2015. They prepared a strictly alternating copolymer of vinyl acetate and SO2 at −70 °C, namely, poly(vinyl acetate-alt-sulfur dioxide), which was found to be responsive to a variety of stimuli, including reactive oxygen species, light, and mechanical force (Fig. 1b) [31]. Mechanistically, an US-induced homolytic bond scission reaction at the S–C bond likely spurred the generation of radicals and thus equilibration to free monomer, leading to depolymerization at low concentrations. Low-Tc poly(vinyl acetate-alt-sulfur dioxide) showed a continuous decrease in molar mass and continued to generate monomers even after the sonication process was terminated. Throughout the sonication process, the temperature of the solution was maintained, and no increase of more than 2 °C was observed. Consequently, the temperature remained within the previously reported range of Tc (−20 °C) and the decomposition temperature (140 °C).
This and the example by Moore and coworkers are particularly appealing since they showcase the depolymerization into the original monomers used for synthesizing the polymers in the first place. However, low-Tc polymers remain without any notable real-world application, limiting their role in closed-loop recycling strategies since they can be mechanochemically degraded but require storage and processing at low temperatures due to their low thermal stability. Conceptually, it may be possible in future endeavors to introduce significantly large alterations in the Tc by mechanochemical gating [32], rendering the polymer thermally stable and processable in the latent state. Therefore, fundamental thermodynamic constraints of the polymerization-depolymerization equilibrium might be overcome (cf. Section “Mechanochemical recycling of bulk polymers to small molecules”).
Mechanochemical gating of degradability
Wang and coworkers followed a different approach in 2020 when they implemented the protection of a base-cleavable lactone with a cyclobutane mechanophore (Fig. 1c) [33]. While the lactone was also base-sensitive in the protected form, saponification without antecedent scission of the mechanophore did not result in chain cleavage. The application of US to a solution of poly(cyclobutane lactone) generated alkylic esters, which degraded upon methanolysis in the presence of Bu4NOH. Almost simultaneously published and conceptually similar was the approach by Craig and coworkers, who relied on the cyclobutane protection of an acid-sensitive ketal functionality (Fig. 1d) [34]. In the nonactivated form, the hydrolysis of the ketal moieties did not lead to chain scission, while after US-induced cleavage of the cyclobutane mechanophores, CF3COOH induced fragmentation to the corresponding olefinic alcohols and the α,β-unsaturated γ-lactone.
Craig and coworkers then improved this mechanochemically gated degradability approach in 2020 to a point where no external addition of a degradation agent was needed after the latent mechanophores were activated (Fig. 1e) [35]. They achieved this by appending the hydrolyzing substituents directly to a cyclobutene mechanophore, effectively producing a poly(cyclobutene diol). Upon US-induced ring opening, the alcohol termini underwent intramolecular 5-exo-trig lactonization, thereby depolymerizing those chain segments that contained the activated mechanophore. Most notably, Craig and coworkers showed for the first time that this was also in principle possible by using a typical compounding twin screw extruder. Using an extruder increased the rate of the lactonization process, matching the expectation due to the simultaneously increased temperature. While several conceptual challenges remained, this showed that depolymerization mechanochemistry in widely available and scalable extruders is possible for adapting to industrial plastic recycling.
In 2023, Wang and coworkers then reported the combination of a strong mechanochemical gating function and a low-Tc polymer (Fig. 1f) [36]. Therefore, they prepared a masked poly(2,5-dihydrofuran) including cyclobutane mechanophores that can switch from high-Tc to low-Tc on demand. The ring-opening metathesis polymerization of the starting monomer using a 2nd generation Grubbs catalyst afforded the corresponding high-Tc polymer at room temperature. Upon US application, these were ring-opened and reformed the native poly(2,5-dihydrofuran) backbone, which was prone to depolymerization to 2,5-dihydrofuran by processing it at 30 °C for 6 h in the presence of a 2nd generation Grubbs catalyst. Most notably, the authors also attempted extrusion and compression to prove the scalability of their approach leading to no significant mechanophore activation. However, when ball milling a crosslinked film of the masked poly(2,5-dihydrofuran), they found considerable mechanophore activation, constituting a promising outlook for further convergence in polymer and trituration mechanochemistry. Although they observed the highest degradation via sonication, the technique is self-limiting for sustainable polymer degradation in terms of scalability due to the necessity of using large amounts of solvent.
Challenges and opportunities
Overall, several challenges for the development of polymers that are mechanochemically degradable by design remain. First, the conceptual conundrum is that the degradation trigger must be protected with another stimulus that is orthogonal to the environmental usage parameters in which the polymer is applied. Second, until now, mechanical force was only used as the gating stimulus and not as the gated stimulus. Effectively, mechanochemistry has only been an auxiliary technology to date. It may be possible to use different stimuli to gate mechanochemistry [37, 38] as the actual stimulus, which would then widen the scope of possible depolymerization and recycling techniques.
Moreover, the scalability of the mechanophore principle into commodity polymers remains elusive. Mechanophores are expensive, more suitable for use in the production of high-technology goods, and deprive most commercial polymers of their beneficial physicochemical properties. Either mechanophore design must be radically simplified or nonlinear, e.g., catalytic processes must be implemented where very little mechanophore activation spurs complete depolymerization reactions. Commodity polymers could also be transformed into mechanically gated degradable polymers through postpolymerization functionalization or via copolymerization of commercial and mechanophore-based monomers to diminish the cost. The aspect of nonlinearity is also relevant to mechanochemical conversion. Designs relying on quantitative mechanophore conversion to unmask the latent degradable site suffer from incomplete degradation and depolymerization since the critical force threshold can only be overcome in the central region of the linear polymer [39]. Thus, a significant portion of the parent polymer remained in the presented non-Tc-based depolymerization reactions. Last, no developed methods until now show compatibility with existing waste streams, and all would require the roll-out of de novo developed polymer materials.
Computational elucidation of mechanochemical reactivity
The goal of computational or quantum mechanochemistry is to understand the molecular mechanisms of the bond breaking and formation processes that are involved in the degradation of polymers. This can help to improve degradability by either designing new potential mechanophores or investigating the most likely breaking point upon trituration. The methods described in this section are based on quantum chemical investigations of molecular systems by means of (approximately) solving the electronic Schrödinger equation. Currently, this is done by using density functional theory (DFT), which gives a good compromise between accuracy and computational cost. To evaluate whether a compound can be mechanically activated, different computational approaches have been developed. They can be based on isometric or isotensional approaches and will be described in the following section.
Isometric simulations
Isometric simulations are based on simulating external forces as a geometrical constraint. This allows for a systematic investigation of molecular properties depending on a geometric parameter. The most commonly used method of this subclass, Constrained Geometries simulate External Force (CoGEF), is based on subsequent minimizations, where the system is allowed to relax except for the atoms on which an extrinsic force is supposed to act [40, 41]. With each optimization, the distance between the two atoms is gradually increased. As a result, the potential energy along the displacement from the minimum structure is obtained, and the maximal force necessary to activate the mechanophore can be received as the slope of the resulting energy-displacement plot (Fig. 2). The method is efficient and easy to implement, which has spread its use in the last decade. A recently published exhaustive study showed that CoGEF predicts the mechanochemical product correctly in 88% of the more than one hundred studied mechanophores [42].
Schematic representation of the CoGEF method. a The dissociation of a C–C bond in polypropylene (PP) is shown. b The change in potential energy with respect to a displacement of the pulling atoms (outermost atoms of the model PP) is shown. The derivative of the potential energy along the displacement corresponds to the force necessary to break the bond
The CoGEF method can be understood as a static picture of the mechanochemical process, i.e., thermal and dynamic effects are neglected. This leads to two drawbacks: (i) As alluded before, some reactions are not described correctly, particularly in cases where the scissile bond is not aligned with the pulling vector, such as for “flex-activated” mechanophores [43,44,45] or so-called head-to-tail dimers [42]. (ii) The maximal force obtained by CoGEF, Fmax, although shown to correlate somewhat with the experimentally determined forces from single-molecule force spectroscopy (SMFS), is overestimating the experimental force, as no thermal effects are considered [46].
Isotensional simulations
In mechanochemical experiments, the increase in force acting on a system is slow compared to the molecular motion. Therefore, it can be assumed that the external force is constant on the timescale of chemical reactions and events, i.e., that the system behaves adiabatically with respect to the change in force. This enables computational protocols of isotensional simulations where the external force is a mere parameter F0, which differs in how the force is incorporated.
According to Bell’s theory, the activation energy of a mechanochemical reaction, \({G}_{{{{{{\rm{A}}}}}}}\), is linearly dependent on the strength of the applied force, F0, and \(\Delta R\), which is the change in the distance of the atoms at which an external force is applied from the reactant to the transition state (Eq. (1)) [47].
Here, \({G}_{{{{{{\rm{A}}}}}}}^{0}\) is the activation free energy without external force. While Bell’s theory assumes a linear dependence of the activation barrier on the external force, this is often not sufficiently accurate, and an extended Bell theory was suggested going beyond the linear term and including second-order corrections based on the curvature of the potential energy [48]. These quadratic terms proved necessary to describe the force dependence of the ring-opening reaction of benzocyclobutenes correctly [48]. Both original and extended Bell theory have in common that the external force is added after reactant and transition structures are obtained without external force, i.e., the ab initio potential energy surface constructed from quantum chemical computations. This post hoc inclusion of mechanical force requires that the stationary points that are essential for the mechanochemical reaction can be located without external force. This is not the case for dissociation reactions or some Woodward–Hoffmann forbidden reactions, where the transition structures are not optimizable without external force [49].
To be able to investigate mechanochemical reaction pathways that are qualitatively different from the thermal reaction pathways without external force, the mechanical force needs to be included explicitly. The application of a constant (curl-free) external force is mathematically equivalent to a change in the potential energy surface (PES) of the specific compound and therefore leads to the creation of a Force-Modified Potential Energy Surface (FMPES) [50]. The FMPES is constructed by a modification of the forces resulting from the quantum chemical ab initio calculations and is used in computational mechanochemistry rather than the ab initio potential energy surface, Vab initio.
In the EFEI (External Force is Explicitly Included) approach, one variant of constructing the FMPES, the external force F0 multiplied by the distance ΔR between the atoms being pulled is subtracted from the ab initio energy Vab initio to determine the effective, force-dependent energy VFMPES (Eq. (2)).
The bold, upright \({{{{{\bf{x}}}}}}\) denotes the 3N–6-dimensional vector of the internal degrees of freedom of the molecular system. The difference from Bell’s model is that \({V}_{{{{{{\rm{EFEI}}}}}}}\left({{{{{\bf{x}}}}}},{F}_{0}\right)\), i.e., the 3N–6-dimensional FMPES, is now used to perform geometry and saddle point optimizations and for other techniques. When molecular dynamics (MD) simulations are performed on an FMPES, the term steered molecular dynamics (SMD) is used to denote the use of an external force.
The FMPES can differ significantly from the non-modified potential energy surface. Not only lowers the external force the mechanochemical reaction barriers or even makes them disappear but also allows for new minimum structures to form or disappear. As a result, the mechanochemical reaction mechanism can deviate qualitatively from the thermal reaction mechanism, as the extrinsic force can enable new reaction pathways [50, 51]. For example, transition state structures can be trapped, such that the ring-opened form of gDCC becomes a minimum structure [52]. Additionally, the force-dependent product distribution caused by isomerization reactions of (biradical) intermediates could be explained by means of FMPES computations in combination with SMD [53].
A typical force dependence of a chemical reaction is displayed in Fig. 3b, where the effective potential energy against the reaction coordinate is shown for an exothermic reaction without an external force and with the application of a medium and a strong force. As the force increases, the potential energy surface becomes increasingly deformed, and the transition state of the reaction is further lowered in energy. The reaction rate constant k can be estimated by the Eyring Eq. (3),
where kB is Boltzmann’s constant, h is Planck’s constant, R is the ideal gas constant, and T is the temperature. In general, the rate constant becomes larger when increasing the magnitude of the external force F0 for mechanically activatable reactions. Therefore, at high enough forces, the activation barrier GA(F0) is low enough that the lifetime of the molecular system corresponds to the timescale of the respective experiments. In Fig. 3c, the force dependence of the activation energy is shown. The gray area marks the range where the activation energy was reduced by an external force so that the lifetime of the reaction is short enough for the reaction to take place [49, 50]. This approach includes thermal effects in the computations of the activation barriers and thus differs from CoGEF computations, which require the potential energy barrier to completely vanish to predict a mechanochemical reaction [42].
a Schematic illustration of the effect of an extrinsic force on the ring opening of cyclobutene. b Increasing force alters the reaction path on a FMPES, such that the activation energy is reduced. c The increasing force leads to a lowered activation barrier. The gray area denotes the energy region where a (mechano-)chemical reaction proceeds with a lifetime in the order of the time of the experiment
Regarding polymer degradation, the FMPES method can be used to simulate external forces acting on the molecular system. In general, the FMPES method is suitable for describing mechanochemical reactions occurring during ball milling, but the direction of the external force vector is not uniquely defined. In any case, the application of an extrinsic force on a polymer chain will lead to fragmentation.
To assess where commodity polymers degrade when subjected to mechanical stress, the possible breaking points in the main chain need to be identified, and force-dependent barrier heights need to be computed and compared. For the search for possible breaking points in the main chain, automated reaction discovery methods can be used in cases where the polymer becomes more complex and more than one or two different bonds might be broken. Recently, Maeda and coworkers published a study in which they modified the Artificial Force Induced Reaction (AFIR) method, an established method for reaction discovery [54] that they developed [55], to predict the mechanochemical reactivity of various systems [56]. In that study, in addition to known mechanophores, the degradation of commodity polymers was investigated to determine where bond scission appears under mechanical stress (cf. Figure 4 and Table 1 below for a selection of investigated polymers). The rather simple polymers polyethylene (PE), PS, and polypropylene (PP) have just one possible breaking point. Polysulfone (PSU), on the other hand, can break in two places: either a carbon‒carbon bond is broken or, alternatively, a carbon-sulfur bond scission takes place [56]. The study proceeded by investigating the influence of tacticity on the mechanochemical degradability of commodity polymers, showing that the force needed to degrade syndiotactic PP is approximately 370 pN higher than for isotactic PP (Table 1).
Selection of recently examined polymers [56]. The bonds that are cleaved mechanochemically are marked in red. a PE, b PP, c PS, and d PSU
Macroscopic models for mechanochemical bond scission
Apart from the quantum mechanical approach described above to calculate reaction rates, empirical models were developed to quantify the speed of reaction. Mechanochemical bond scission in polymer backbones is described by the Thermally Activated Barrier to Scission (TABS) model by Odell and Keller [57,58,59,60]. While the TABS model was developed for strain in flow fields occurring in polymer solutions, Zhurkov and Bueche investigated the fracture of polymer chains when exposing solid polymer samples to mechanical stress [61,62,63]. Their observations are in agreement with the TABS model. Zhurkov formulated a modification of the Arrhenius equation for the rate constant k for chain cleavage when a macroscopic stress σ is applied (Eq. (4)) with the preexponential factor k0, the activation energy barrier EA and an activation volume α, which relates the macroscopic stress to the force acting on the bond to be broken [64].
This is similar to Bell’s model, which describes the change in a chemical reaction barrier by an external force at the molecular level, as shown in Eq. (1). Accordingly, Eq. (4) can be seen as a macroscopic version of Bell’s theory. A comparison of experimental values obtained for the parameters in Eq. (4), in comparison to computational values, such as force-dependent reaction barriers, could yield insight into the force transfer from the macroscopic to the molecular level, i.e., the activation volume α.
Challenges and opportunities
While the basic principles of force-dependent quantum chemical investigations are established and the abovementioned methods work well for a wide range of linearly acting forces in polymer mechanochemistry, modeling the effect of trituration remains a challenge since the direction of the forces acting on the substrate is not well defined. For example, in ball milling, balls crush the molecular probe, exerting forces in random directions at every collision. While some studies claim that the mechanical effect of ball milling only results in a change in the local concentration and/or the dielectric constant [65], others used more sophisticated approaches such as hydrostatic pressure or selected molecular distortions to mimic uniaxial compression to model the influence of trituration [66]. Moreover, the direction as well as the magnitude of the external force vector acting on a molecular level is unknown. Thus, it remains unclear how to connect molecular-level simulations to the macroscopic forces exerted by ball milling, and a comprehensive comparison of different approaches with experimental results is needed to build methods able to predict the outcome of trituration experiments.
Another challenge for computational methods is to explain and quantify the reactions occurring after the primary mechanochemical reactions. In particular, the nonselective reactivity of the resulting radicals leads to a plethora of unforeseen chemical reactions, such as hydrogen migration, initiation of autoxidation, and radical transfer reactions. Automated methods of computational reaction discovery can explore these degradation pathways. However, this is a computationally demanding endeavor, as the resulting chemical reaction network can easily consist of hundreds of molecular species. Furthermore, surrounding molecules, such as solvent or other polymer segments, must be included in the simulations to find intermolecular reactions, such as reactions with O2, which can lead to accelerated degradation via autoxidation [67]. The resulting chemical reaction network can be used to construct a microkinetic model to predict the most likely reaction pathway and products upon the degradation mechanisms depending on temperature, external force, concentration of environmental molecules, and other parameters. This can help to optimize conditions for an optimal degradation efficiency and reveal possible catalytic mechanisms. Among various methods for automated reaction discovery, the ab initio nanoreactor approach might be a suitable tool for the discovery of radical reactions [68, 69]. Additionally, the recent extension of the AFIR method was able to find all possible scission reactions of a crosslinking agent upon mechanochemical activation [56], making this method a promising tool for the systematic exploration of mechanochemical reaction networks. However, further development of computational methods is needed to make molecular reaction discovery more efficient and construct more complete chemical reaction networks.
Mechanochemical recycling of bulk polymers to small molecules
While the coupling of mechanochemistry and design-for-recycling can provide the next generation of sustainable materials, this concept is not applicable to the >8 Gt of plastic already produced [70]. Nonetheless, mechanochemistry offers potential for the chemical recycling of these bulk polymers by transforming them into their monomers or other raw materials needed in the chemical industry. The mechanochemical liberation of small molecules from polymers with a C–C backbone was already shown by Regel and coworkers in 1967, who observed that poly(methyl methacrylate) (PMMA), PS, and PP release volatile products, among others monomers, after rupture of the backbone bonds due to mechanical load [71]. This effect has recently received attention as a strategy for depolymerization of PS and PMMA [72, 73]. However, especially for most produced plastics, i.e., polyolefins such as PE and PP, key challenges have to be addressed: (i) Control over the selectivity is challenging due to the absence of predefined breaking points. (ii) Polyolefins are difficult to depolymerize due to their low glass transition temperatures Tg. (iii) The existence of a limiting molar mass below which no chain cleavage can occur seems to prohibit full depolymerization. (iv) Finally, providing the thermodynamic driving force for depolymerization is challenging.
(i) Polyolefins offer no functional groups in the backbone that can be targeted for selective (mechano-)chemical cleavage strategies. During the purely thermal conversion of PP by pyrolysis above 400 °C, over 500 products are obtained [74]. While lower temperatures during mechanoactivation could help direct selectivity due to the lowered reactivity of active intermediates, the fundamental challenge of controlling where the polymer is cleaved and how subsequent reactions proceed remains. Condensation polymers such as polyethylene terephthalate (PET), on the other hand, offer heteroatom functionalities in the backbone, which can be targeted as breaking points for selective mechanochemical cleavage strategies, such as those observed in thermochemical recycling by solvolysis [75,76,77]. Recent examples have demonstrated the mechanochemical depolymerization of PET with NaOH [78, 79] and of the biopolymers cellulose [80], chitosan [81], and chitin [82] under acidic conditions.
(ii) The degradation rate constant, a measure of chain scission frequency, when milling PMMA and PS is strongly dependent on the Tg, with a high Tg indicating high cleavage rates [83]. However, the Tg of polyolefins is far below room temperature, which likely leads to a lower impact efficiency (Table 1). Thus, it is not surprising that the mechanochemical recycling of PE and PP is very challenging and, to the best of our knowledge, has not yet been reported.
(iii) Full depolymerization of polymers requires the mechanochemical activation of very short-chain polymers. To enable polymer backbone cleavage, however, macroscopic forces have to be translated to submolecular forces along multiple length scales. To explain how macroscopic stress “arrives” at molecular C–C bonds, Zhurkov and Bueche proposed that chain entanglements play a crucial role [61,62,63]. While entanglement keeps the polymer chains from slipping past each other, allowing for effective force transfer, a higher number of entanglements also allows the forces to dissipate along a larger number of bonds, which leads to lower stress concentrations [63]. However, the exact nature of force application in milling processes remains unclear (cf. Section “Macroscopic models for mechanochemical bond scission”). It is clear, however, that polymeric interactions are a prerequisite for mechanochemical bond cleavage and that hydrocarbons below a certain degree of polymerization (Xn,lim) are “inert” to ball milling [63, 84]. Values for Xn,lim have been reported for PS [85,86,87], PE [84], PMMA [88] (Table 1), and polyvinylpyrrolidone (Xn,lim = 75) [89]. Sohma and Sakaguchi explained this phenomenon in terms of a balance between interatomic forces (i.e., chemical bonds) and intermolecular forces (i.e., van der Waals forces) [63, 90]. The presence of a limiting molar mass under which no chain cleavage takes place has important implications for the mechanochemical recycling of bulk polymers since it poses a fundamental challenge to full depolymerization.
(iv) To design a successful mechanochemical conversion strategy, kinetic and thermodynamic considerations must be made. As discussed in Section “Macroscopic models for mechanochemical bond scission”, macroscopic mechanical stress is introduced into the plastic material and translated to mechanical bonds, thereby increasing the energy of the starting material and lowering the experienced activation energy barrier to bond cleavage. This accelerates the kinetics of generating radicals as active chain ends, which can then undergo depolymerization to shorter hydrocarbons (Fig. 5). However, a thermodynamic driving force must exist to drive depolymerization of this active chain end. The ceiling temperature Tc is the temperature above which depolymerization is thermodynamically favored over polymerization. When comparing different polymers, a lower Tc indicates a higher thermodynamic drive to depolymerize. The high Tc of polyolefins is thus unfavorable (Table 1). This is also illustrated in Table 2 using dodecane as a model compound: cracking to hexane and hexene is only favored above 500 °C. Therefore, pathways beyond cracking deserve attention to drive thermodynamics. For example, aromatization creates an entropic driving force, but the reaction remains unfavorable under ambient conditions unless it is coupled with the consumption of H2 in a sufficiently favorable reaction. In contrast, negative free energies of reaction are obtained for the hydrogenolysis or oxidative cracking of dodecane. Indeed, hydrogenolysis [91], tandem hydrogenolysis/aromatization [92], and mechanocatalytic oxidative cracking [78] have been used as driving forces for PE conversion in the literature.
Subsequent reactions of mechanoradicals formed after bond cleavage by applying a force F: Spin trapping for electron spin resonance (ESR)-spectroscopic analysis, depolymerization to generate monomers, and the formation of branches and crosslinks via hydrogen migration and disproportionation. The mechanism is drawn for PE, but the considerations are also applicable to PP, PS, and PMMA
Mechanocatalysis can provide a kinetic driving force, while oxidative strategies can provide a thermodynamic driving force. For example, in their report on mechanochemical conversion of PS to its monomer, Balema, Luzinov, and coworkers proposed that O2 in concert with steel shavings is crucial to the production of monomers via a mechanism involving oxidation of C-centered chain-end radicals to peroxy radicals [73]. Further work by Sievers and coworkers determined that styrene is also produced in the absence of O2, but its presence increases the initial rate of monomer production and leads to the formation of oxygenated products. An example of explicit catalytic oxidation to cause cracking of polyolefinic chains using mechanochemistry is the work of Sievers and coworkers, where PE was processed mechanocatalytically with a peroxide-based Fenton system to randomly oxidize some of the carbon atoms in the polymer backbone [93]. The resulting functional groups facilitate subsequent mechanochemical C–C cleavage, leading to shorter chains. These strategies could also address the challenge of a limiting degree of polymerization.
Compared to thermochemical scission, mechanochemical approaches offer unique advantages to bypass classical equilibrium thermodynamic limitations. Conceptually, mechanical energy, such as that producing during ball milling, is supplied in a highly localized manner and enables chemical transformations without bulk heating, thereby allowing control over the selectivity. While cleavage and fast primary radical reactions occur under purely mechanochemical and low-temperature conditions, respectively, undesired follow-up reactions, as discussed in the following section, can be suppressed. Furthermore, mechanical forces continuously remove products from the impact zone, and gaseous products can be continuously removed from the milling chamber, which drives equilibria in a favorable way.
Reactions of mechanoradicals
The chain cleavage yields two mechanoradicals, and their fate is governed by the same principles as observed in radical polymerizations. Subsequent reactions either preserve or annihilate the radical functionality (Fig. 5). The desired pathway in the context of chemical recycling is β-scission, yielding the monomer. However, direct scission products are unstable primary or secondary radicals, and H abstraction and transfer of the radical functionality to more stabilized positions within or outside of the same polymer chain can induce crosslinking and branching [94]. Termination of the reactive radical functionality can occur via the combination of chain radicals and disproportionation [60]. Disproportionation of two polymer radicals leads to one saturated and one unsaturated chain end [95]. Disproportionation and recombination are two competing reactions, but recombination is often faster due to its lower activation energy. Similar to thermal degradation, a radical cage model was hypothesized, where after homolytic cleavage of a C–C backbone bond, two radicals reside relatively close to one another. This can lead to prompt termination. However, in mechanochemistry, ball collisions can free radicals from the cage, thereby overcoming the slow diffusion observed for thermal reactions, which could decrease the rate of recombination [96].
ESR spectroscopy of mechanoradicals
To generate insight into the depolymerization mechanism and direct selectivity patterns, exact knowledge of the active intermediates is necessary. To this end, electron spin resonance (ESR) spectroscopy is a key technique to study even low concentrations of radicals. Splitting patterns occurring due to hyperfine interactions of radicals and atomic nuclei allow us to characterize the products of mechanochemical bond cleavage [90]. ESR spectroscopy can be used directly on milled plastics without further additives [73, 97, 98]. Therefore, Sohma and coworkers identified the initial scission products produced by sawing PE at 77 K (Fig. 6a) and confirmed that mechanical fracture yields primary radicals [99]. To counter the limited lifetime of mechanoradicals, such as those generated from PE and PP [100], at room temperature, spin traps can be used to scavenge these transient species [63]. These typically nitroso- or nitrone-derived molecules (Fig. 6b) trap the transient radical as a spin adduct (i.e., nitroxide radical, Fig. 5) [100, 101]. Sohma and coworkers used this method to identify the mechanoradicals generated upon ultrasonic irradiation of PMMA and PS solutions [102]. At even higher temperatures (230 °C), more stable hindered amine light stabilizers were used as radical traps to prove the formation of mechanoradicals during the extrusion of PP in air (Fig. 6c, d) [103]. In another approach, Dondi, Buttafava, Faucitano and coworkers added the radical (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) to styrene butadiene rubber to study the yields of mechanoradicals under mechanical treatment. TEMPO combines with generated mechanoradicals, leading to a hydroxylamine ether without radical functionality, and the decline in radical concentration was followed by ESR [104].
a Identification of primary scission radicals during the fracture of PE: Measured (above, observed after sawing of PE) and simulated (below, assuming a primary chain-end radical) ESR spectra. Adapted from Sohma and coworkers [99]. b Commonly used agents in ESR spectroscopic studies of polymer fracture: (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO, b1), pentamethylnitrosobenzene (b2), and bis(2,2,6,6-tetramethyl-4-piperidyl-1-oxyl) sebacate (a hindered amine light stabilizer, b3). c ESR spectrum after extrusion of PP under air with b3 as a radical stabilizer at 230 °C. Reprinted from Sohma [103]. d Comparison of ESR intensity as a measure of the amount of radicals generated at 230 °C with and without mechanical activation (extrusion) under air with b3. Reprinted from Sohma [103]. Graphics are reproduced with permission from Springer Nature
Challenges and opportunities
Historically, mechanochemical bond scission has been treated as an unwanted effect in polymer processing and production, and research has focused on preventing or controlling these effects. Now, in the quest for tapping polymers as sustainable hydrocarbon feedstock for the chemical industry, this knowledge base is instrumental to retailoring mechanochemistry for the purposeful degradation of polymers to small molecules. The advantage of this approach over other chemical recycling strategies is that the energy supplied is highly localized, which bypasses the need for bulk heating. The low bulk temperature also provides a handle to control unwanted reactions following initial C–C cleavage that lead to cyclization, branching, and recombination during pyrolysis. In addition, polymers are not cleaved randomly under mechanical force but have a preference for cleavage near the middle of the chain and for cleavage of longer chains over shorter chains [39].
The mechanochemical approach, however, still suffers from low activity, especially for very thermodynamically stable high-Tc polymers, such as PE and PP (cf. Section “Mechanophores for the accelerated degradation of polymers”). In addition to mechanochemically gated reactivity, catalytic strategies lowering the activation energy for C–C bond cleavage and stabilizing chain-end radicals promoting depolymerization over termination via recombination and novel reactor geometries inducing higher stress in the plastic materials could tackle this. Another challenge is the presence of a limiting molar mass, which seems to prohibit full depolymerization to monomers. However, well-defined oligomers of the limiting degree of polymerization could be an interesting byproduct that is easily separated from liquid- or gas-phase monomers.
While industrial chemical processes are mostly driven by burning fossil fuels for heating, ball milling provides a unique conceptual advantage of tapping green electricity or even mechanical energy directly to drive chemical conversions. This would make the conversion of mechanical energy from, for example, hydropower to electricity, unnecessary and mitigates associated conversion losses. In addition, a ball milling process could be less vulnerable to impurities in plastic waste. For example, chlorine, a common impurity stemming from polyvinylchloride, leads to HCl formation and reactor fouling in high-temperature plastic conversion, such as pyrolysis, and ball milling slightly above room temperature could create a condition under which HCl is much less corrosive [105, 106].
Analysis of mechanochemistry: ex situ, in situ, and online
Spectroscopic analysis is key to understanding the mechanism of reactions during ball milling. However, sampling during milling disturbs the reaction by adding air and removing volatile components [107, 108]. In addition, the remaining sample amount is reduced, changing the impact and kinetics of the reaction [109,110,111]. To circumvent this, a variety of online and in situ analytical techniques have been implemented to monitor mechanochemical reactions during ball milling. While they have not been applied to polymer ball milling, their applicability to study polymer transformations is discussed below.
Online and in situ ball milling
Both in situ Raman spectroscopy and X-ray diffraction (XRD) have been applied to ball milling [112]. For example, a hand-held Raman probe was positioned close to a custom-made PMMA milling container placed in a conventional ball mill [107, 109] to study the reaction of cadmium chloride and cyanoguanidine to form coordination polymers [109]. The placement of all components is critical for recording a clear signal and depends on the geometry of the milling container. For a horizontal shaker ball mill, the probe is ideally placed below the jar and focused close to the inside of the container wall where the material continuously falls (Fig. 7a) [107]. However, not all ball mills allow for such placement. The InSolido Technologies IST 636 was used in the above example, while a plate at the bottom of the Retsch MM400 prohibits this. In organic systems, Raman signals from PMMA may overlap with those from the analyte, and other milling container materials may need to be considered because Weidenthaler and coworkers detected small amounts of decomposition products of their PMMA container during milling [113].
In situ analysis configurations for reaction monitoring during milling. a Raman spectroscopy where a probe is placed perpendicular to the motion of the shaker ball mill. Care is taken to ensure that the focus of the probe is within the PMMA container. Horizontal arrows indicate the direction of shaking. reprinted with permission from Lukin and coworkers [107]. b A custom-made milling geometry used at the Scherrer Institute beamline leads to narrower diffraction peaks and less background than the setup similar to the one shown in Panel (a) for in situ XRD. The custom-made container has a steel inner milling chamber and an X-ray transparent outer analysis chamber (pink). The shaking can be set to either vertical or horizontal movement (blue double arrow), while the jar is also rotated (blue curled arrow). Adapted from Casati and coworkers [115]. c Configuration for simultaneous XRD, Raman spectroscopy, and thermography during milling. The vibratory mill shakes a PMMA jar vertically. Care is taken to ensure that the various techniques do not interfere with each other. Adapted from Emmerling and coworkers [122]
While Raman spectroscopy is performed at the lab bench with a probe, XRD often requires a synchrotron light source. For in situ operation, high flux is necessary for the scattered beam to exit the container. Examples of reactions studied by this method are the mechanosynthesis of zeolitic imidazolate frameworks, inorganic metathesis reactions, and disulfide exchange reactions [110, 114]. Several beamlines allow PMMA containers to be directly placed in the beam path (Fig. 7b) but often require modifications to the horizontal shaker mill [110, 113, 114]. Due to the semicrystalline nature of PMMA, some scattering is observed from the container and may also be observed from the milling spheres. A dedicated setup developed by the Scherrer Institute circumvents this issue with a double chambered milling/analysis container, where milling occurs in a metal half-jar that allows the product but not the grinding spheres to fall into a probing chamber made from X-ray transparent foil through which the beam passes (Fig. 7c) [115].
To allow for solid-state nuclear magnetic resonance (ssNMR) measurements during ball milling, Wüllen and coworkers built a ball mill inside an ssNMR tube by attaching a rotational motor that caused translational motion moving a polyoxymethylene sample container (sample chamber 10 × 8 mm) back and forth by 1 cm [116]. As a test reaction, they studied the formation of zinc phenylphosphonate from zinc acetate and phenylphosphonic acid milled with 50 ZrO2 milling balls (1 mm diameter). Initial results were obtained using single-pulse acquisition experiments, which necessitated deconvolution. Additionally, the concurrent shaking of the milling container in the NMR interfered with the measurement; thus, they alternated quickly between milling and measuring. In another ssNMR technique, magic angle spinning (MAS), the sample is rotated rapidly around a magic angle of 54.74°, leading to an elimination of the orientational dependence of anisotropic interactions of powders in NMR [117]. Attempts by Wüllen and coworkers to use MAS led to problems due to mechanochemical reactions being induced by spinning, even without the presence of milling media, indicating that in situ milling with spheres could be driven by MAS motion. However, as Wiegand, Bolm, and coworkers recently reviewed, very small milling media (0.1 mm) need to be used to avoid an imbalance of the rotor [118]. Such small spheres, however, would not create enough force to directly cleave C–C bonds, which is necessary for polyolefin degradation.
Gases evolving from mechanochemical depolymerization are also of interest. Schüth and coworkers used online gas IR analysis to monitor the presence of CO and CO2 when oxidizing CO over Cu catalysts in a ball mill [119]. Other gas analytical techniques are also possible, with Sievers and coworkers using online mass spectrometry to analyze the formation of ammonia from H2 and N2 over TiN [120].
Finally, multiple analytical techniques can be used simultaneously. Halasz, Užarević, and coworkers combined XRD and Raman to quantify polymorphs in cocrystalline species [121], while Emmerling and coworkers and Hernández and coworkers methods for monitoring both organic and inorganic reactions with a combination of XRD, Raman, and thermography using an IR camera [122, 123] (Fig. 7c).
Spectroscopy of depolymerization reactions
Ex situ measurements performed during polymer processing and polymerization reactions can inspire future studies during ball milling to understand depolymerization. In Raman and IR, vinyl stretches are observed between 1645 and 1640 cm−1 and can be used to track disproportionation [124, 125]. In the case of the oxidative degradation or depolymerization of PET, the C = O stretches (ca. 1700 cm−1) and C–O stretches (1050–1250 cm−1) can be used to monitor the extent of the reaction [93, 124]. IR and Raman can also be used to detect bond angle distortions under mechanical loading [64]. XRD can be used to follow transitions between crystalline and amorphous states as well as between multiple crystalline states, for example, the formation of a monoclinic phase from an orthorhombic phase in strained PE [126,127,128,129]. In NMR, changes such as alkene formation and branching could be monitored.
Single impact studies
An approach to rigorously probe the reaction environment during mechanochemical reactions is to examine physical and chemical transformations on a thin piece of reactant material that is subjected to a single collision. Any characterization technique that is compatible with microscopy or spatial mapping can be leveraged for physical or chemical analysis of the impacted area to correlate the extent of chemical changes to the parameters of the collision. The kinematic properties of the impact can be obtained via motion analysis with a high-speed camera.
For binary interfacial reactions such as between PET and NaOH, a dispersion of the latter can be coated from a suspension onto a thin film of the former to create a model interface. Compression of the film sample during collision will press the two layers into each other to induce solid-solid mixing and the desired mechanochemical reaction.
A simple setup to carry out single-collision experiments involves a stationary flat base on which the film sample is mounted and a milling ball that is dropped from an appropriate height to hit the film sample. This concept is inspired in part by setups reported in the thermographic study of heat transfer during mechanical impact between solid bodies [130, 131]. The simplicity of the setup allows for more sophisticated environmental controls and monitoring instruments to be implemented without tremendous effort.
Challenges and opportunities
While the in situ spectroscopy techniques highlighted here have been successfully used for a variety of mechanochemical transformations, their applicability to polymer milling has yet to be demonstrated. Due to the simplicity of the setup, in situ Raman spectroscopy is the most likely to be implemented, while infrared spectroscopy measurements are more difficult due to the need to measure in transmission. These transmission measurements require two probes to be placed less than a centimeter opposite each other and would thus penetrate into the milling jar. In addition, the sticky nature of polymers could acerbate problems observed with other milled powders, such as accumulation at the wall of the milling jar, leading to a false signal if the spectroscopic probe is focused there. A focus closer into the milling jar might not yield the desired result either as not enough sample might be in the beam and scattering due to the milling spheres is likely to disturb the measurement. New dedicated ball milling chambers for Raman, IR, and XRD measurements are thus needed, especially if quantitative measurements are desired. In addition, NMR measurements are unlikely to be performed on polymer milling directly. Apart from the methods discussed above, UV‒Vis spectroscopy could be interesting. This technique was applied for tracking radicals formed during ultrasonication of PMMA, stabilizing the radicals with spin trap 2,2-diphenyl-1-picrylhydrazyl (DPPH) [132]. In addition, in situ fluorescence spectroscopy could be combined with spin probes that show increased fluorescence intensity upon trapping, as was previously shown for ultrasonication and bulk polymer processes [133,134,135,136]. These technologies additionally have the advantage of being mechanophore-free, as opposed to usually employed optical force probes [137], thereby exhibiting universal and scalable character (cf. Section “Mechanophores for the accelerated degradation of polymers”).
Despite these difficulties, this observation would yield crucial insight into the propagation and termination of mechanoradicals. This knowledge is necessary for the targeted promotion of propagation reactions such as β-scission, which would yield the desired monomer formation and thus selective depolymerization. Finally, combining single impact and bulk in situ studies could be used to elucidate the effect of the collision of the grinding spheres with each other and the wall from other macroscopic effects such as mixing, agglomeration of polymer at the milling jar wall or heat dissipation.
Conclusion
Polymer mechanochemistry shows tremendous potential to tackle the polymer waste streams of today and the future. In particular, the amalgamation of polymer and trituration mechanochemistry presents itself as the only currently available method to exert force on polymers at scale with the necessary energy input. As an example, Rinaldi and coworkers analyzed the energy requirement for H2SO4-catalyzed hydrolysis of cellulose to water-soluble oligosaccharides in Simolayer ball mills and reported that the energy requirement decreased from ca. 200–9.6 MWh t−1 as the process was scaled from 1 g–1 kg with milling times of 2.0–2.5 h [138]. They suggested that this economy of scale can be extrapolated to even larger mills. While rigorous process models would be needed to assess the energy efficiency of mechanochemical plastic depolymerization at the ton scale, it appears that the requirements will not be prohibitive.
However, many fundamental and grueling barriers must be overcome to eventually apply the polymer mechanochemistry principle for sustainability. This starts with the high cost of mechanophore production, which will require the development of future concepts with inexpensive or even mechanophore-free approaches. In addition, the thermodynamic and kinetic aspects of highly endergonic and self-limiting depolymerization reactions must be addressed by catalytic or other nonlinear approaches decoupling the thermal from the mechanochemical reaction pathway. Computational approaches will play a major role in rationalizing and unifying the understanding of mechanochemical reactions but will also contribute to the discovery of entirely new force-steered reactions of polymers. This will be accompanied by new methods to observe polymer mechanochemical reactions, which have their own technical difficulties in decoupling highly abrasive mechanochemical conditions from delicate analytic instruments.
While these obstacles require the communal efforts of the brightest minds for eventual success, the authors of this review are convinced that the polymer mechanochemistry principle shows no irresolvable dilemmas that would sabotage its eventual application at scale to play a significant role in a sustainable and circular polymer future.
References
Staudinger H, Heuer W. Über hochpolymere Verbindungen, 93. Mitteil.: Über das Zerreißen der Faden-Moleküle des Poly-styrols. Berichte Dtsch Chem Ges B Ser. 1934;67:1159–64.
Kauzmann W, Eyring H. The viscous flow of large. Molecules J Am Chem Soc. 1940;62:3113–25.
O’Neill RT, Boulatov R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat Rev Chem. 2021;5:148–67.
Chen Y, Mellot G, van Luijk D, Creton C, Sijbesma RP. Mechanochemical tools for polymer materials. Chem Soc Rev. 2021;50:4100–40.
De Bo G. Polymer mechanochemistry and the emergence of the mechanophore concept. Macromolecules. 2020;53:7615–7.
Willis-Fox N, Watchorn-Rokutan E, Rognin E, Daly R. Technology pull: scale-up of polymeric mechanochemical force sensors. Trends Chem. 2023;5:415–31.
Klok H-A, Herrmann A, Göstl R. Force ahead: emerging applications and opportunities of polymer mechanochemistry. ACS Polym Au. 2022;2:208–12.
Dubach FFC, Ellenbroek WG, Storm C. How accurately do mechanophores report on bond scission in soft polymer materials? J Polym Sci. 2021;59:1188–99.
James SL, Friščić T. Mechanochemistry. Chem Soc Rev. 2013;42:7494–6.
Horie K, Barón M, Fox RB, He J, Hess M, Kahovec J, et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl Chem. 2004;76:889–906.
Friščić T, Trask A V, Jones W, Motherwell WDS. Screening for inclusion compounds and systematic construction of three-component solids by liquid-assisted grinding. Angew Chem Int Ed. 2006;45:7546–50.
James SL, Adams CJ, Bolm C, Braga D, Collier P, Friščić T, et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev. 2012;41:413–47.
Xuan M, Schumacher C, Bolm C, Göstl R, Herrmann A. The mechanochemical synthesis and activation of carbon-rich π-conjugated materials. Adv Sci. 2022;9:2105497.
Krusenbaum A, Grätz S, Tigineh GT, Borchardt L, Kim JG. The mechanochemical synthesis of polymers. Chem Soc Rev. 2022;51:2873–905.
Zhou J, Hsu T-G, Wang J. Mechanochemical degradation and recycling of synthetic polymers. Angew Chem Int Ed. 2023;62:e202300768.
Akbulatov S, Boulatov R. Experimental polymer mechanochemistry and its interpretational frameworks. ChemPhysChem. 2017;18:1422–50.
Shelef O, Gnaim S, Shabat D. Self-immolative polymers: an emerging class of degradable materials with distinct disassembly. Profiles J Am Chem Soc. 2021;143:21177–88.
Peterson GI, Larsen MB, Boydston AJ. Controlled depolymerization: stimuli-responsive self-immolative polymers. Macromolecules. 2012;45:7317–28.
Black AL, Orlicki JA, Craig SL. Mechanochemically triggered bond formation in solid-state polymers. J Mater Chem. 2011;21:8460–5.
Ramirez ALB, Kean ZS, Orlicki JA, Champhekar M, Elsakr SM, Krause WE, et al. Mechanochemical strengthening of a synthetic polymer in response to typically destructive shear forces. Nat Chem. 2013;5:757–61.
Verstraeten F, Göstl R, Sijbesma RP. Stress-induced colouration and crosslinking of polymeric materials by mechanochemical formation of triphenylimidazolyl radicals. Chem Commun. 2016;52:8608–11.
Zhang H, Gao F, Cao X, Li Y, Xu Y, Weng W, et al. Mechanochromism and mechanical-force-triggered cross-linking from a single reactive moiety incorporated into polymer chains. Angew Chem Int Ed. 2016;55:3040–4.
Pan Y, Zhang H, Xu P, Tian Y, Wang C, Xiang S, et al. A mechanochemical reaction cascade for controlling load-strengthening of a mechanochromic polymer. Angew Chem Int Ed. 2020;59:21980–5.
Ducrot E, Chen Y, Bulters M, Sijbesma RP, Creton C. Toughening elastomers with sacrificial bonds and watching them break. Science. 2014;344:186–9.
Izak-Nau E, Braun S, Pich A, Göstl R. Mechanically resistant poly(N-vinylcaprolactam) microgels with sacrificial supramolecular catechin hydrogen bonds. Adv Sci. 2022;9:2104004.
Wang S, Hu Y, Kouznetsova TB, Sapir L, Chen D, Herzog-Arbeitman A, et al. Facile mechanochemical cycloreversion of polymer cross-linkers enhances tear resistance. Science. 2023;380:1248–52.
Diesendruck CE, Peterson GI, Kulik HJ, Kaitz JA, Mar BD, May PA, et al. Mechanically triggered heterolytic unzipping of a low-ceiling-temperature polymer. Nat Chem. 2014;6:623–8.
Cravotto G, Gaudino EC, Cintas P. On the mechanochemical activation by ultrasound. Chem Soc Rev. 2013;42:7521–34.
Martínez RF, Cravotto G, Cintas P. Organic sonochemistry: a chemist’s timely perspective on mechanisms and reactivity. J Org Chem. 2021;86:13833–56.
Tricker AW, Samaras G, Hebisch KL, Realff MJ, Sievers C. Hot spot generation, reactivity, and decay in mechanochemical reactors. Chem Eng J. 2020;382:122954.
Kumar K, Goodwin AP. Alternating sulfone copolymers depolymerize in response to both chemical and mechanical stimuli. ACS Macro Lett. 2015;4:907–11.
Hu H, Ma Z, Jia X. Reaction cascades in polymer mechanochemistry. Mater Chem Front. 2020;4:3115–29.
Hsu T-G, Zhou J, Su H-W, Schrage BR, Ziegler CJ, Wang J. A polymer with “locked” degradability: superior backbone stability and accessible degradability enabled by mechanophore installation. J Am Chem Soc. 2020;142:2100–4.
Lin Y, Kouznetsova TB, Craig SL. Mechanically gated degradable polymers. J Am Chem Soc. 2020;142:2105–9.
Lin Y, Kouznetsova TB, Chang C-C, Craig SL. Enhanced polymer mechanical degradation through mechanochemically unveiled lactonization. Nat Commun. 2020;11:4987.
Hsu T-G, Liu S, Guan X, Yoon S, Zhou J, Chen W-Y, et al. Mechanochemically accessing a challenging-to-synthesize depolymerizable polymer. Nat. Commun. 2023;14:225.
Kida J, Imato K, Goseki R, Aoki D, Morimoto M, Otsuka H. The photoregulation of a mechanochemical polymer scission. Nat. Commun. 2018;9:3504.
He S, Schog S, Chen Y, Ji Y, Panitz S, Richtering W, et al. Photoinduced mechanical cloaking of diarylethene-crosslinked microgels. Adv Mater. 2023;35:2305845.
Lenhardt JM, Ramirez Black, Lee AL, Kouznetsova B, Craig TB, Mechanistic SL. Insights into the sonochemical activation of multimechanophore cyclopropanated polybutadiene polymers. Macromolecules. 2015;48:6396–403.
Szyja B, Pidko E, Groote R, Hensen E, Sijbesma R. DFT Study on mechanochemical bond breaking in COGEF and Molecular Dynamics simulations. Procedia Comput Sci. 2011;4:1167–76.
Beyer MK. The mechanical strength of a covalent bond calculated by density functional theory. J Chem Phys. 2000;112:7307–12.
Klein IM, Husic CC, Kovács DP, Choquette NJ, Robb MJ. Validation of the CoGEF method as a predictive tool for polymer mechanochemistry. J Am Chem Soc. 2020;142:16364–81.
Larsen MB, Boydston AJ. “Flex-activated” mechanophores: using polymer mechanochemistry to direct bond bending activation. J Am Chem Soc. 2013;135:8189–92.
Larsen MB, Boydston AJ. Successive mechanochemical activation and small molecule release in an elastomeric. Material J Am Chem Soc. 2014;136:1276–9.
Shen H, Larsen MB, Roessler AG, Zimmerman PM, Boydston AJ. Mechanochemical release of N-heterocyclic carbenes from flex-activated mechanophores. Angew Chem Int Ed. 2021;60:13559–63.
Stauch T, Dreuw A. Advances in quantum mechanochemistry: electronic structure methods and force analysis. Chem Rev. 2016;116:14137–80.
Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–27.
Konda SSM, Brantley JN, Bielawski CW, Makarov DE. Chemical reactions modulated by mechanical stress: extended Bell theory. J Chem Phys. 2011;135:164103.
Brown CL, Bowser BH, Meisner J, Kouznetsova TB, Seritan S, Martinez TJ, et al. Substituent effects in mechanochemical allowed and forbidden cyclobutene ring-opening reactions. J Am Chem Soc. 2021;143:3846–55.
Ong MT, Leiding J, Tao H, Virshup AM, Martínez TJ. First principles dynamics and minimum energy pathways for mechanochemical ring opening of cyclobutene. J Am Chem Soc. 2009;131:6377–9.
Wollenhaupt M, Schran C, Krupička M, Marx D. Force-induced catastrophes on energy landscapes: mechanochemical manipulation of downhill and uphill bifurcations explains the ring-opening selectivity of cyclopropanes. ChemPhysChem. 2018;19:837–47.
Lenhardt JM, Ong MT, Choe R, Evenhuis CR, Martinez TJ, Craig SL. Trapping a diradical transition state by mechanochemical polymer extension. Science. 2010;329:1057–60.
Liu Y, Holm S, Meisner J, Jia Y, Wu Q, Woods TJ, et al. Flyby reaction trajectories: Chemical dynamics under extrinsic force. Science. 2021;373:208–12.
Ismail I, Chantreau Majerus R, Habershon S. Graph-driven reaction discovery: progress challenges, and future opportunities. J Phys Chem A. 2022;126:7051–69.
Maeda S, Harabuchi Y, Takagi M, Taketsugu T, Morokuma K. Artificial force induced reaction (AFIR) method for exploring quantum chemical potential energy surfaces. Chem Rec. 2016;16:2232–48.
Jiang J, Kubota K, Jin M, Wang ZJ, Nakajima T, Ito H, et al. Computational exploration of polymer mechanochemistry: quantitation of activation force and systematic discovery of reaction sites utilizing two forces. Preprint at 2022 https://doi.org/10.26434/chemrxiv-2022-fr09l.
Odell JA, Keller A, Muller AJ. Thermomechanical degradation of macromolecules. Colloid Polym Sci. 1992;270:307–24.
Odell JA, Keller A, Rabin Y. Flow-induced scission of isolated macromolecules. J Chem Phys. 1988;88:4022–8.
Odell JA, Keller A. Flow-induced chain fracture of isolated linear macromolecules in solution. J Polym Sci Part B Polym Phys. 1986;24:1889–916.
Caruso MM, Davis DA, Shen Q, Odom SA, Sottos NR, White SR, et al. Mechanically-induced chemical changes in polymeric materials. Chem Rev. 2009;109:5755–98.
Zhurkov SN. Kinetic concept of the strength of solids. Int J Fract Mech. 1965;1:311–23.
Bueche F. Tensile strength of plastics below the glass temperature. J Appl Phys. 1957;28:784–7.
Sohma J. Mechanochemistry of polymers. Prog Polym Sci. 1989;14:451–596.
Zhurkov SN, Korsukov VE. Atomic mechanism of fracture of solid polymers. J Polym Sci Polym Phys Ed. 1974;12:385–98.
Pladevall BS, de Aguirre A, Maseras F. Understanding ball milling mechanochemical processes with DFT calculations and microkinetic modeling. ChemSusChem. 2021;14:2763–8.
Zholdassov YS, Yuan L, Garcia SR, Kwok RW, Boscoboinik A, Valles DJ, et al. Acceleration of Diels-Alder reactions by mechanical distortion. Science. 2023;380:1053–8.
Keer LD, Steenberge PV, Reyniers M-F, Gryn’ova G, Aitken HM, Coote ML. New mechanism for autoxidation of polyolefins: kinetic Monte Carlo modelling of the role of short-chain branches, molecular oxygen and unsaturated moieties. Polym Chem. 2022;13:3304–14.
Wang L-P, Titov A, McGibbon R, Liu F, Pande VS, Martínez TJ. Discovering chemistry with an ab initio nanoreactor. Nat Chem. 2014;6:1044–8.
Xu R, Meisner J, Chang AM, Thompson KC, Martínez TJ. First principles reaction discovery: from the Schrodinger equation to experimental prediction for methane pyrolysis. Chem Sci. 2023;14:7447–64.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3:e1700782.
Amelin AV, Muinov TM, Pozdnyakov OF, Regel VR. Comparison of the mass spectra of the volatile products liberated from polymers in the course of mechanical destruction and thermal degradation. Polym Mech. 1967;3:54–60.
Jung E, Yim D, Kim H, Peterson GI, Choi T-L. Depolymerization of poly(α-methyl styrene) with ball-mill grinding. J Polym Sci. 2023;61:553–60.
Balema VP, Hlova IZ, Carnahan SL, Seyedi M, Dolotko O, Rossini AJ, et al. Depolymerization of polystyrene under ambient conditions. New J Chem. 2021;45:2935–8.
Vollmer I, Jenks MJF, Roelands MCP, White RJ, Harmelen T, Wild P et al. Beyond mechanical recycling: giving new life to plastic waste. Angew Chem Int Ed. 2020;59:15402–23.
Shojaei B, Abtahi M, Najafi M. Chemical recycling of PET: a stepping‐stone toward sustainability. Polym Adv Technol. 2020;31:2912–38.
Iwaya T, Sasaki M, Goto M. Kinetic analysis for hydrothermal depolymerization of nylon 6. Polym Degrad Stab. 2006;91:1989–95.
Piemonte V, Sabatini S, Gironi F. Chemical recycling of PLA: a great opportunity towards the sustainable development? J Polym Environ. 2013;21:640–7.
Tricker AW, Osibo AA, Chang Y, Kang JX, Ganesan A, Anglou E, et al. Stages and kinetics of mechanochemical depolymerization of poly(ethylene terephthalate) with sodium hydroxide. ACS Sustain Chem Eng. 2022;10:11338–47.
Štrukil V. Highly efficient solid‐state hydrolysis of waste polyethylene terephthalate by mechanochemical milling and vapor‐assisted aging. ChemSusChem. 2021;14:330–8.
Meine N, Rinaldi R, Schüth F. Solvent-free catalytic depolymerization of cellulose to water-soluble oligosaccharides. ChemSusChem. 2012;5:1449–54.
Yang G, Lam E, Moores A. Controlled chitosan molecular weight reduction by mechanochemical and aging-based phosphoric acid hydrolysis. ACS Sustain Chem Eng. 2023;11:7765–74.
Yabushita M, Kobayashi H, Kuroki K, Ito S, Fukuoka A. Catalytic depolymerization of chitin with retention of N-acetyl group. ChemSusChem. 2015;8:3760–3.
Peterson GI, Ko W, Hwang YJ, Choi TL. Mechanochemical degradation of amorphous polymers with ball-mill grinding: influence of the glass transition temperature. Macromolecules. 2020;53:7795–802.
Sakaguchi M, Sohma J. ESR evidence for main-chain scission produced by mechanical fracture of polymers at low temperature. J Polym Sci Polym Phys Ed. 1975;13:1233–45.
Vivatpanachart S, Nomura H, Miyahara Y. Cryogenic crushing of polystyrene. J Appl Polym Sci. 1981;26:1485–91.
Kankeo Y, Sugiyama K. Degradation behaviors of polystyrene and polycarbonate by vibration ball mill. J Soc Mater Sci Jpn. 1977;26:1214–7.
Fukunaga K, Kimura M. Decrease of molecular weight of polystyrene by ball-milling. Chem Lett. 1987;16:1503–6.
Kondo S, Sasai Y, Hosaka S, Ishikawa T, Kuzuya M. Kinetic analysis of the mechanolysis of polymethylmethacrylate in the course of vibratory ball milling at various mechanical energy. J Polym Sci Part Polym Chem. 2004;42:4161–7.
Pas T, Bergonzi A, Michiels E, Rousseau F, Schymkowitz J, Koekoekx R, et al.Preparation of amorphous solid dispersions by cryomilling: chemical and physical concerns related to active pharmaceutical ingredients and carriers. Mol Pharm. 2020;17:1001–13.
Sohma J, Sakaguchi M. ESR studies on polymer radicals produced by mechanical destruction and their reactivity. In: New Scientific Aspects. Springer-Verlag; 1976. p. 109–58. https://doi.org/10.1007/BFb0023970.
Rorrer JE, Beckham GT, Román-Leshkov Y. Conversion of polyolefin waste to liquid alkanes with ru-based catalysts under mild conditions. JACS Au. 2021;1:8–12.
Zhang F, Zeng M, Yappert RD, Sun J, Lee Y-H, LaPointe AM, et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science. 2020;370:437–41.
Nguyen VS, Chang Y, Phillips E V, DeWitt JA, Sievers C. Mechanocatalytic oxidative cracking of poly(ethylene) via a heterogeneous fenton process. ACS Sustain Chem Eng. 2023;11:7617–23.
Fordyce P, Devries KL, Fanconi BM. Chain scission and mechanical degradation of polystyrene. Polym Eng Sci. 1984;24:421–7.
Rideal GR, Padget JC. The thermal-mechanical degradation of high density polyethylene. J Polym Sci Polym Symp. 1976;57:1–15.
Butyagin PY. Kinetics and nature of mechanochemical reactions. Russ Chem Rev. 1971;40:901–15.
Sakaguchi M, Kodama S, Edlund O, Sohma J. ESR study on PMMA main chain scission induced by mechanical fracture. J Polym Sci Polym Lett Ed 1974;12:609–13.
Tabata M, Yamakawa H, Takahashi K, Sohma J. ESR studies on the conversion of mechanoradicals of poly(methyl methacrylate) and its self-degradation. Polym Degrad Stab. 1979;1:57–68.
Kawashima T, Shimada S, Kashiwabara H, Sohma J. ESR studies on the molecular mechanisms of fracture of polymers at low temperatures. Polym J. 1973;5:135–43.
Sakaguchi M, Kashiwabara H. Melting and annealing effects on molecular motion of spin-labelled molecules at the polymer chain-end located on the polypropylene surface. Polymer. 1982;23:1594–8.
Tabata M, Hosokawa Y, Watanabe O, Sohma J. Direct evidence for main chain scissions of polymers in solution caused by high speed stirring. Polym J. 1986;18:699–712.
Tabata M, Miyazawa T, Kobayashi O, Sohma J. Direct evidence of main-chain scissions induced by ultrasonic irradiation of benzene solutions of polymers. Chem Phys Lett. 1980;73:178–80.
Sohma J. Mechano-radical formation in polypropylene by an extruder action and its after-effects. Colloid Polym Sci. 1992;270:1060–5.
Dondi D, Zeffiro A, Buttafava A, Marciano C, Bianchi M, Faucitano A. Mechanical degradation of elastomers in the presence of silica and inhibitors using a new design of mechano reactor. Polym Degrad Stab. 2013;98:392–407.
Baláž M, Bujňáková Z, Achimovičová M, Tešinský M, Baláž P. Simultaneous valorization of polyvinyl chloride and eggshell wastes by a semi-industrial mechanochemical approach. Environ Res. 2019;170:332–6.
Lu J, Borjigin S, Kumagai S, Kameda T, Saito Y, Yoshioka T. Practical dechlorination of polyvinyl chloride wastes in NaOH/ethylene glycol using an up-scale ball mill reactor and validation by discrete element method simulations. Waste Manag. 2019;99:31–41.
Lukin S, Užarević K, Halasz I. Raman spectroscopy for real-time and in situ monitoring of mechanochemical milling reactions. Nat Protoc. 2021;16:3492–521.
Štrukil V, Fábián L, Reid DG, Duer MJ, Jackson GJ, Eckert-Maksić M, et al. Towards an environmentally-friendly laboratory: dimensionality and reactivity in the mechanosynthesis of metal–organic compounds. Chem Commun. 2010;46:9191.
Gracin D, Štrukil V, Friščić T, Halasz I, Užarević K. Laboratory real-time and in situ monitoring of mechanochemical milling reactions by Raman spectroscopy. Angew Chem Int Ed. 2014;53:6193–7.
Lampronti GI, Michalchuk AAL, Mazzeo PP, Belenguer AM, Sanders JKM, Bacchi A, et al. Changing the game of time resolved X-ray diffraction on the mechanochemistry playground by downsizing. Nat Commun. 2021;12:6134.
Michalchuk AAL, Emmerling F. Time‐resolved in situ monitoring of mechanochemical reactions. Angew Chem Int Ed. 2022;61:e202117270.
Julien PA, Friščić T. Methods for monitoring milling reactions and mechanistic studies of mechanochemistry: a primer. Cryst. Growth Des. 2022;22:5726–54.
Rathmann T, Petersen H, Reichle S, Schmidt W, Amrute AP, Etter M, et al. In situ synchrotron x-ray diffraction studies monitoring mechanochemical reactions of hard materials: challenges and limitations. Rev Sci Instrum. 2021;92:114102.
Friščić T, Halasz I, Beldon PJ, Belenguer AM, Adams F, Kimber SAJ, et al. Real-time and in situ monitoring of mechanochemical milling reactions. Nat Chem. 2013;5:66–73.
Ban V, Sadikin Y, Lange M, Tumanov N, Filinchuk Y, Černý R, et al. Innovative in situ ball mill for X-ray diffraction. Anal Chem. 2017;89:13176–81.
Schiffmann JG, Emmerling F, Martins ICB, Van Wüllen L. In-situ reaction monitoring of a mechanochemical ball mill reaction with solid state NMR. Solid State Nucl Magn Reson. 2020;109:101687.
Polenova T, Gupta R, Goldbourt A. Magic angle spinning NMR spectroscopy: a versatile technique for structural and dynamic analysis of solid-phase systems. Anal Chem. 2015;87:5458–69.
Silva I, d’Anciães A, Bartalucci E, Bolm C, Wiegand T. Opportunities And challenges in applying solid-state NMR spectroscopy in organic mechanochemistry. Adv Mater. 2023;35:2304092.
Eckert R, Felderhoff M, Schüth F. Preferential carbon monoxide oxidation over copper-based catalysts under in situ ball milling. Angew Chem Int Ed. 2017;56:2445–8.
Tricker AW, Hebisch KL, Buchmann M, Liu Y-H, Rose M, Stavitski E, et al. Mechanocatalytic ammonia synthesis over TiN in transient microenvironments. ACS Energy Lett. 2020;5:3362–7.
Lukin S, Stolar T, Tireli M, Blanco M V, Babić D, Friščić T, et al. Tandem in situ monitoring for quantitative assessment of mechanochemical reactions involving structurally unknown phases. Chem Eur J. 2017;23:13941–9.
Kulla H, Haferkamp S, Akhmetova I, Röllig M, Maierhofer C, Rademann K, et al. In situ investigations of mechanochemical one-pot syntheses. Angew Chem Int Ed. 2018;57:5930–3.
Ardila‐Fierro KJ, Lukin S, Etter M, Užarević K, Halasz I, Bolm C, et al. Direct visualization of a mechanochemically induced molecular rearrangement. Angew Chem Int Ed. 2020;59:13458–62.
Socrates G. Infrared and Raman characteristic group frequencies. Tables and charts. John Wiley & Sons, Ltd; New York; 2001.
Kruse TM, Wong H-W, Broadbelt LJ. Mechanistic modeling of polymer pyrolysis: polypropylene. Macromolecules. 2003;36:9594–607.
Goikhman AS, Kirichenko VI, Budnitskii GA, Korolenko MP, Matsibora NP. X-ray diffraction measurements of the crystallinity of polypropylene fibers. Polym Sci USSR. 1984;26:974–81.
Teare PW, Holmes DR. Extra reflections in the x-ray diffraction pattern of polyethylenes and polymethylenes. J Polym Sci. 1957;24:496–9.
Zamfirova G, Pereña JM, Benavente R, Pérez E, Cerrada ML, Nedkov E. Mechanical properties of ultra high molecular weight polyethylene obtained with different cocatalyst systems. Polym J. 2002;34:125–31.
Vivatpanachart S, Nomura H, Miyahara Y, Kashiwabara H, Sakaguchi M. E.s.r. study of the motion of spin trapped radicals in polyethylene in relation to the morphological changes induced by ball-milling. Polymer. 1981;22:263–7.
Maierhofer C, Krankenhagen R, Röllig M. Application of thermographic testing for the characterization of impact damage during and after impact load. Compos Part B Eng. 2019;173:106899.
Ben-Ammar F, Kaviany M, Barber JR. Heat transfer during impact. Int J Heat Mass Transf. 1992;35:1495–506.
Wang F, Burck M, Diesendruck CE. Following homolytic mechanochemical kinetics with a pyrenyl nitrone spin trap. ACS Macro Lett. 2017;6:42–5.
Kubota K, Toyoshima N, Miura D, Jiang J, Maeda S, Jin M, et al. Introduction of a luminophore into generic polymers via mechanoradical coupling with a prefluorescent reagent. Angew Chem Int Ed. 2021;60:16003–8.
Yamamoto T, Kato S, Aoki D, Otsuka H. A diarylacetonitrile as a molecular probe for the detection of polymeric mechanoradicals in the bulk state through a radical chain-transfer mechanism. Angew Chem Int Ed. 2021;60:2680–3.
Zheng Y, Jiang J, Jin M, Miura D, Lu FX, Kubota K, et al. In situ and real-time visualization of mechanochemical damage in double-network hydrogels by prefluorescent probe via oxygen-relayed radical trapping. J Am Chem Soc. 2023;145:7376–89.
Matsuda T, Kawakami R, Nakajima T, Gong JP. Crack tip field of a double-network gel: visualization of covalent bond scission through mechanoradical polymerization. Macromolecules. 2020;53:8787–95.
He S, Stratigaki M, Centeno SP, Dreuw A, Göstl R. Tailoring the properties of optical force probes for polymer mechanochemistry. Chem Eur J. 2021;27:15889–97.
Kaufman Rechulski MD, Käldström M, Richter U, Schüth F, Rinaldi R. Mechanocatalytic depolymerization of lignocellulose performed on hectogram and kilogram scales. Ind Eng Chem Res. 2015;54:4581–92.
Hiemenz PC, Lodge TP. Polymer chemistry. 2nd ed. Taylor & Francis; Boca Raton, London, New York; 2007.
Lechner MD, Gehrke K, Nordmeier EH. Makromolekulare chemie. Berlin: Springer; 2014. https://doi.org/10.1007/978-3-642-41769-6.
Acknowledgements
Funding is acknowledged from the German Research Foundation for 503981124 (R.G.), the Federal Ministry of Education and Research for 031B1148A (S.A., R.G.), the Dutch Research Council for VI.Veni.202.191 (I.V.) and OCENW.XS22.1.093 (C.L.S.), and the National Science Foundation for 2028998 (G.C., C.S.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aydonat, S., Hergesell, A.H., Seitzinger, C.L. et al. Leveraging mechanochemistry for sustainable polymer degradation. Polym J 56, 249–268 (2024). https://doi.org/10.1038/s41428-023-00863-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00863-9