Abstract
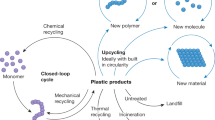
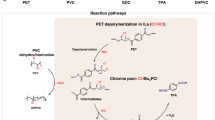
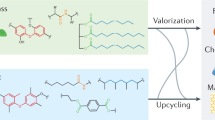
Plastics pollution is causing an environmental crisis, prompting the development of new approaches for recycling, and upcycling. Here, we review challenges and opportunities in chemical and biological catalysis for plastics deconstruction, recycling, and upcycling. We stress the need for rigorous characterization and use of widely available substrates, such that catalyst performance can be compared across studies. Where appropriate, we draw parallels between catalysis on biomass and plastics, as both substrates are low-value, solid, recalcitrant polymers. Innovations in catalyst design and reaction engineering are needed to overcome kinetic and thermodynamic limitations of plastics deconstruction. Either chemical and biological catalysts will need to act interfacially, where catalysts function at a solid surface, or polymers will need to be solubilized or processed to smaller intermediates to facilitate improved catalyst–substrate interaction. Overall, developing catalyst-driven technologies for plastics deconstruction and upcycling is critical to incentivize improved plastics reclamation and reduce the severe global burden of plastic waste.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Andrady, A. L. Marine Anthropogenic Litter (eds. Bergmann, M. et al.) 57–72 (Springer International Publishing, 2015).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015).
Nicholson, S. R., Rorrer, N. A., Carpenter, A. C. & Beckham, G. T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 5, 673–686 (2021). This article estimates the supply-chain energy requirements and greenhouse gas emissions associated with US plastics consumption, providing a foundation on which to compare emerging technologies in the field of the circular plastics economy.
The New Plastics Economy—Rethinking The Future of Plastics (Ellen Macarthur Foundation & McKinsey & Company, 2016).
Ragaert, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Garcia, J. M. & Robertson, M. L. The future of plastics recycling. Science 358, 870–872 (2017).
Himmel, M. E. et al. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 (2007).
Chamas, A. et al. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 8, 3494–3511 (2020). This perspective summarizes the existing literature on the rates of plastic degradation in the environment and implements a specific surface degradation rate as a metric to evaluate the degradation half-lives of a variety of plastics under various environmental conditions.
Rudroff, F. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 1, 12–22 (2018).
Ebnesajjad, S. in Chemical Resistance of Engineering Thermoplastics (eds. Baur, E. et al.) xiii–xxv (William Andrew Publishing, 2016).
Gibbs, J. H. Nature of the glass transition in polymers. J. Chem. Phys. 25, 185–186 (1956).
Fox, T. G. & Flory, P. J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. 14, 315–319 (1954).
Teator, A. J. & Leibfarth, F. A. Catalyst-controlled stereoselective cationic polymerization of vinyl ethers. Science 363, 1439–1443 (2019).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Mohan, T. P., George, A. P. & Kanny, K. Combined effect of isophthalic acid and polyethylene glycol in polyethylene terephthalate polymer on thermal, mechanical, and gas transport properties. J. Appl. Polym. Sci. 126, 536–543 (2012).
Gabriel, C. Influence of molecular structure on rheological properties of polyethylenes. Rheol. Acta 37, 7–20 (1998).
Rennie, A. R. in Mechanical Properties and Testing of Polymers: An A–Z Reference (ed. Swallowe, G. M.) 248 (Springer Netherlands, 1999).
Kamelian, F. S., Saljoughi, E., Nasirabadi, P. S. & Mousavi, S. M. Modifications and research potentials of acrylonitrile/butadiene/styrene (ABS) membranes: a review. Polym. Compos. 39, 2835–2846 (2017).
Hahladakis, J. N., Velis, C. A., Weber, R., Iacovidou, E. & Purnell, P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 344, 179–199 (2018).
Daniels, P. H. A brief overview of theories of PVC plasticization and methods used to evaluate PVC–plasticizer interaction. J. Vinyl Addit. Techn. 15, 219–223 (2009).
Andrady, A. L. & Neal, M. A. Applications and societal benefits of plastics. Phil. Trans. R. Soc. Lond. B Biol. Sci. 364, 1977–1984 (2009).
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D. & Templeton, D. W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 58, 9043–9053 (2010).
Gohil, R. M. Morphology–property relationship in oriented PET films: microstructural reorganization during heat treatment. J. Appl. Polym. Sci. 52, 925–944 (1994).
Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 395, 1589–1611 (2009).
Nielen, M. W. F. Maldi time-of-flight mass spectrometry of synthetic polymers. Mass Spectrom. Rev. 18, 309–344 (1999).
Malkin, A. Y. A. The state of the art in the rheology of polymers: achievements and challenges. Polym. Sci. Ser. A 51, 80–102 (2009).
Ko, Y. C., Ratner, B. D. & Hoffman, A. S. Characterization of hydrophilic—hydrophobic polymeric surfaces by contact angle measurements. J. Colloid Interface Sci. 82, 25–37 (1981).
Argyle, M. D. & Bartholomew, C. H. Heterogeneous catalyst deactivation and regeneration: a review. Catalysts 5, 145–269 (2015).
Saalwächter, K. in New Developments in NMR (eds. Zhang, R. et al.) 1–22 (Royal Society of Chemistry, 2019).
Camacho, W. & Karlsson, S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic waste. Polym. Eng. Sci. 41, 1626–1635 (2001).
Hiller, W. et al. On-line coupling of high temperature GPC and 1H NMR for the analysis of polymers. J. Magn. Reson. 183, 290–302 (2006).
Boldon, L., Laliberte, F. & Liu, L. Review of the fundamental theories behind small angle X-ray scattering, molecular dynamics simulations, and relevant integrated application. Nano Rev. 6, 25661 (2015).
Materazzi, S. Thermogravimetry—infrared spectroscopy (TG-FTIR) coupled analysis. Appl. Spectrosc. Rev. 32, 385–404 (1997).
Smolders, K. & Baeyens, J. Thermal degradation of PMMA in fluidised beds. Waste Manag. 24, 849–857 (2004).
Pifer, A. & Sen, A. Chemical recycling of plastics to useful organic compounds by oxidative degradation. Angew. Chem. Int. Ed. 37, 3306–3308 (1998). This communication reports the oxidative depolymerization of a variety of polymers (polystyrene, polyethylene, polypropylene, poly(methyl methacrylate) and so on) using oxygen and nitrogen oxides, successfully obtaining small molecules from these plastics under relatively mild conditions.
Takuma, K., Uemichi, Y. & Ayame, A. Product distribution from catalytic degradation of polyethylene over H-gallosilicate. Appl. Catal. A Gen. 192, 273–280 (2000).
Renzini, M. S., Sedran, U. & Pierella, L. B. H-ZSM-11 and Zn-ZSM-11 zeolites and their applications in the catalytic transformation of LDPE. J. Anal. Appl. Pyrol. 86, 215–220 (2009).
Beydoun, K. & Klankermayer, J. Efficient plastic waste recycling to value-added products by integrated biomass processing. ChemSusChem 13, 488–492 (2020).
Jehanno, C., Pérez-Madrigal, M. M., Demarteau, J., Sardon, H. & Dove, A. P. Organocatalysis for depolymerisation. Polym. Chem. 10, 172–186 (2018).
Anuar Sharuddin, S. D., Abnisa, F., Wan Daud, W. M. A. & Aroua, M. K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 115, 308–326 (2016).
Huang, J. B., Zeng, G. S., Li, X. S., Cheng, X. C. & Tong, H. Theoretical studies on bond dissociation enthalpies for model compounds of typical plastic polymers. IOP Conf. Ser. Earth Environ. Sci. 167, 012029 (2018).
Westerhout, R. W. J., Waanders, J. & Kuipers, J. A. M. Kinetics of the low-temperature pyrolysis of polyethene, polypropene, and polystyrene modeling, experimental determination, and comparison with literature models and data. Ind. Eng. Chem. Res. 36, 1955–1964 (1997).
Wong, S. L., Ngadi, N., Abdullah, T. A. T. & Inuwa, I. M. Current state and future prospects of plastic waste as source of fuel: a review. Renew. Sust. Energy Rev. 50, 1167–1180 (2015).
Aishwarya, K. N. & Sindhu, N. Microwave assisted pyrolysis of plastic waste. Proc. Technol. 25, 990–997 (2016).
Ding, K. et al. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 196, 1316–1325 (2019).
Goto, M. Chemical recycling of plastics using sub- and supercritical fluids. J. Supercrit. Fluids 47, 500–507 (2009).
Kamimura, A. & Yamamoto, S. An efficient method to depolymerize polyamide plastics: a new use of ionic liquids. Org. Lett. 9, 2533–2535 (2007). This article describes the depolymerization of polyamides to produce ϵ-caprolactam, which was catalysed by ionic liquids that also served as the reaction solvent, demonstrating a relatively simple strategy to produce small molecules from polyamide plastics.
Whiston, K., Forsyth, S. & Seddon, K. R. Ionic liquid solvents and a process for the depolymerization of polyamides. WIPO patent WO2009016378A3 (2009).
Liu, F., Cui, X., Yu, S., Li, Z. & Ge, X. Hydrolysis reaction of poly(ethylene terephthalate) using ionic liquids as solvent and catalyst. J. Appl. Polym. Sci. 114, 3561–3565 (2009).
Song, X. et al. Hydrolysis of polycarbonate catalyzed by ionic liquid [Bmim][Ac]. J. Hazard. Mater. 244–245, 204–208 (2013).
Kamimura, A., Yamamoto, S. & Yamada, K. Depolymerization of unsaturated polyesters and waste fiber-reinforced plastics by using ionic liquids: the use of microwaves to accelerate the reaction rate. ChemSusChem 4, 644–649 (2011).
Bockstal, L., Berchem, T., Schmetz, Q. & Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: a review. J. Clean. Prod. 236, 117574 (2019).
Jablonský, M., Škulcová, A. & Šima, J. Use of deep eutectic solvents in polymer chemistry—a review. Molecules 24, 3978 (2019).
Lucarini, M. & Pedulli, G. F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 39, 2106–2119 (2010).
Ügdüler, S., VanGeem, K. M., Roosen, M., Delbeke, E. I. P. & De Meester, S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manag. 104, 148–182 (2020).
Mosier, N. et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96, 673–686 (2005).
Vandenburg, H. J. et al. Critical review: analytical extraction of additives from polymers. Analyst 122, 101R–116R (1997).
Zhao, Y.-B., Lv, X.-D. & Ni, H.-G. Solvent-based separation and recycling of waste plastics: a review. Chemosphere 209, 707–720 (2018).
Anastas, P. & Eghbali, N. Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312 (2010).
Sheldon, R. A. The E factor: fifteen years on. Green Chem. 9, 1273–1283 (2007).
Biddy, M. J. et al. The techno-economic basis for coproduct manufacturing to enable hydrocarbon fuel production from lignocellulosic biomass. ACS Sustain. Chem. Eng. 4, 3196–3211 (2016).
Li, K. & Sun, Y. Electrocatalytic upgrading of biomass-derived intermediate compounds to value-added products. Chem. Eur. J. 24, 18258–18270 (2018).
Jiang, T. et al. STEP polymer degradation: solar thermo-coupled electrochemical depolymerization of plastics to generate useful fuel plus abundant hydrogen. Sol. Energy Mater. Sol. Cells 204, 110208 (2020).
Chen, T. et al. Photo-induced depolymerisation: recent advances and future challenges. ChemPhotoChem 3, 1059–1076 (2019).
Schneider, J. et al. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 114, 9919–9986 (2014).
Thomas, R. T. & Sandhyarani, N. Enhancement in the photocatalytic degradation of low density polyethylene–TiO2 nanocomposite films under solar irradiation. RSC Adv. 3, 14080–14087 (2013).
Uekert, T., Kasap, H. & Reisner, E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst. J. Am. Chem. Soc. 141, 15201–15210 (2019).
Achilias, D. S. in Microwave-Assisted Polymer Synthesis (eds. Hoogenboom, R. et al.) 309–346 (Springer International Publishing, 2016).
Achilias, D. S. et al. Glycolytic depolymerization of PET waste in a microwave reactor. J. Appl. Polym. Sci. 118, 3066–3073 (2010).
Siddiqui, M. N. et al. Hydrolytic depolymerization of PET in a microwave reactor. Macromol. Mater. Eng. 295, 575–584 (2010).
Klun, U. & Kržan, A. Rapid microwave induced depolymerization of polyamide-6. Polymer 41, 4361–4365 (2000).
Song, X., Zhang, S. & Zhang, D. Catalysis investigation of PET depolymerization under metal oxides by microwave irradiation. J. Appl. Polym. Sci. 117, 3155–3159 (2010).
Bäckström, E., Odelius, K. & Hakkarainen, M. Trash to treasure: microwave-assisted conversion of polyethylene to functional chemicals. Ind. Eng. Chem. Res. 56, 14814–14821 (2017). This article describes a method for depolymerization of LDPE into small dicarboxylic acids in a dilute aqueous nitric acid solution by employing microwave irradiation to promote the degradation, shortening the reaction time compared with conventional heating, and resulting in high product yields.
Alberti, C., Figueira, R., Hofmann, M., Koschke, S. & Enthaler, S. Chemical recycling of end-of-life polyamide 6 via ring closing depolymerization. ChemistrySelect 4, 12638–12642 (2019).
Caruso, M. M. et al. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Amirjalayer, S., Fuchs, H. & Marx, D. Understanding the mechanocatalytic conversion of biomass: a low-energy one-step reaction mechanism by applying mechanical force. Angew. Chem. Int. Ed. 58, 5232–5235 (2019).
Hick, S. M. et al. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 12, 468–474 (2010).
Shende, V. S., Saptal, V. B. & Bhanage, B. M. Recent advances utilized in the recycling of homogeneous catalysis. Chem. Rec. 19, 2022–2043 (2019).
Janssen, M., Müller, C. & Vogt, D. Recent advances in the recycling of homogeneous catalysts using membrane separation. Green Chem. 13, 2247–2257 (2011).
McBride, K., Gaide, T., Vorholt, A., Behr, A. & Sundmacher, K. Thermomorphic solvent selection for homogeneous catalyst recovery based on COSMO-RS. Chem. Eng. Process. 99, 97–106 (2016).
Van Leeuwen, P. W. N. M. Decomposition pathways of homogeneous catalysts. Appl. Catal. A Gen. 212, 61–81 (2001).
Polizzi, K. M., Bommarius, A. S., Broering, J. M. & Chaparro-Riggers, J. F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 11, 220–225 (2007).
Bommarius, A. S. & Paye, M. F. Stabilizing biocatalysts. Chem. Soc. Rev. 42, 6534–6565 (2013).
Jones, C. W. On the stability and recyclability of supported metal–ligand complex catalysts: myths, misconceptions and critical research needs. Top. Catal. 53, 942–952 (2010).
Huang, Z. et al. Highly active and recyclable heterogeneous iridium pincer catalysts for transfer dehydrogenation of alkanes. Adv. Synth. Catal. 351, 188–206 (2009).
Jia, X., Qin, C., Friedberger, T., Guan, Z. & Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2, e1501591 (2016). This article reports on a method for the degradation of polyethylene to light alkanes through tandem catalytic cross-alkane metathesis by employing a homogeneous Ir and heterogeneous Re catalyst, with the ability to tailor selectivity towards alkanes in the diesel range or waxes by changing the identity of the Ir complex.
Barth, M. et al. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 11, 1082–1087 (2016).
Lopez, G., Artetxe, M., Amutio, M., Bilbao, J. & Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sust. Energy Rev. 73, 346–368 (2017).
Kunwar, B., Cheng, H. N., Chandrashekaran, S. R. & Sharma, B. K. Plastics to fuel: a review. Renew. Sust. Energy Rev. 54, 421–428 (2016).
Al-Salem, S. M., Lettieri, P. & Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag. 29, 2625–2643 (2009).
Al-Salem, S. M., Antelava, A., Constantinou, A., Manos, G. & Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 197, 177–198 (2017).
Serrano, D. P., Aguado, J. & Escola, J. M. Developing advanced catalysts for the conversion of polyolefinic waste plastics into fuels and chemicals. ACS Catal. 2, 1924–1941 (2012).
Schenk, N. J., Biesbroek, A., Heeres, A. & Heeres, H. J. Process for the preparation of aromatic compounds. US patent US20170247617A1 (2017).
Ding, W., Liang, J. & Anderson, L. L. Hydrocracking and hydroisomerization of high-density polyethylene and waste plastic over zeolite and silica–alumina-supported Ni and Ni–Mo sulfides. Energy Fuels 11, 1219–1224 (1997).
Escola, J. M. et al. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl. Catal. B 106, 405–415 (2011).
Aguado, J. Deactivation and regeneration of a Ni supported hierarchical Beta zeolite catalyst used in the hydroreforming of the oil produced by LDPE thermal cracking. Fuel 109, 679–686 (2013).
Sriningsih, W. et al. Fuel production from LDPE plastic waste over natural zeolite supported Ni, Ni–Mo, Co and Co–Mo metals. Procedia Environ. Sci. 20, 215–224 (2014).
Bin Jumah, A., Anbumuthu, V., Tedstone, A. A. & Garforth, A. A. Catalyzing the hydrocracking of low density polyethylene. Ind. Eng. Chem. Res. 58, 20601–20609 (2019).
Akah, A., Hernandez-Martinez, J., Rallan, C. & Garforth, A. A. Enhanced feedstock recycling of post-consumer plastic waste. Chem. Eng. Trans. 43, 2395–2400 (2015).
Hibbitts, D. D., Flaherty, D. W. & Iglesia, E. Role of branching on the rate and mechanism of C–C cleavage in alkanes on metal surfaces. ACS Catal. 6, 469–482 (2016).
Rorrer, J. E., Beckham, G. T. & Román-Leshkov, Y. Conversion of polyolefin waste to liquid alkanes with Ru-based catalysts under mild conditions. JACS Au. 1, 8–12 (2020). This article reports a strategy for the depolymerization of LDPE through hydrogenolysis catalysed by Ru/C, with control over the product selectivity ranging from long-chain liquid alkanes to methane.
Tennakoon, A. et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 3, 893–901 (2020). This article describes the depolymerization of HDPE using an elegant materials design approach with a mesoporous shell/active site/core catalyst system in which Pt catalysts are situated at the base of mesopores that can encapsulate hydrocarbon chains, mimicking the active site architecture of enzyme-catalysed processes to obtain a narrow distribution of diesel and lubricant-range alkane products.
Celik, G. et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 5, 1795–1803 (2019).
Dufaud, V. & Basset, J.-M. Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica‐alumina: a step toward polyolefin degradation by the microscopic reverse of Ziegler–Natta polymerization. Angew. Chem. Int. Ed. 37, 806–810 (1998).
Coates, G. W., Hustad, P. D. & Reinartz, S. Catalysts for the living insertion polymerization of alkenes: access to new polyolefin architectures using Ziegler–Natta chemistry. Angew. Chem. Int. Ed. 41, 2236–2257 (2002).
Shamiri, A. et al. The influence of Ziegler–Natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 7, 5069–5108 (2014).
Westhues, S., Idel, J. & Klankermayer, J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts. Sci. Adv. 4, eaat9669 (2018). This article reports the hydrogenolysis of poly(lactic acid), PET and polycarbonate using a molecular Ru catalyst, achieving high conversion and selectivity to small molecule diol products with high tolerance towards polymer additives, as demonstrated by effective depolymerization of a variety of consumer-grade plastics.
Krall, M. E. et al. Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium(ii) PNN pincer complexes. Chem. Commun. 50, 4884–4887 (2014).
Ellis, L. D. et al. Tandem heterogeneous catalysis for polyethylene depolymerization via an olefin-intermediate process. ACS Sustain. Chem. Eng. 9, 623–628 (2021). This article describes a method for depolymerizing polyethylene to liquid alkanes using tandem dehydrogenation and olefin cross-metathesis with fully heterogeneous Pt and Re catalysts, and broadly describes how this strategy is probably one of many potential examples of an olefin-intermediate process.
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020). This report outlines an exciting, solvent-free strategy to convert polyethylene to alkylaromatics using a tandem hydrogenolysis/aromatization approach by coupling the exothermic hydrogenolysis and endothermic aromatization to achieve a thermodynamically favourable process.
Ahmad, N., Abnisa, F. & Wan Daud, W. M. A. Potential use of natural rubber to produce liquid fuels using hydrous pyrolysis—a review. RSC Adv. 6, 68906–68921 (2016).
Smith, R. F., Boothroyd, S. C., Thompson, R. L. & Khosravi, E. A facile route for rubber breakdown via cross metathesis reactions. Green Chem. 18, 3448–3455 (2016).
Bidange, J., Fischmeister, C. & Bruneau, C. Ethenolysis: a green catalytic tool to cleave carbon–carbon double bonds. Chem. Eur. J. 22, 12226–12244 (2016).
Partenheimer, W. Valuable oxygenates by aerobic oxidation of polymers using metal/bromide homogeneous catalysts. Catal. Today 81, 117–135 (2003).
Tomás, R. A. F., Bordado, J. C. M. & Gomes, J. F. P. p-Xylene oxidation to terephthalic acid: a literature review oriented toward process optimization and development. Chem. Rev. 113, 7421–7469 (2013).
Murakami, M. & Matsuda, T. Metal-catalysed cleavage of carbon–carbon bonds. ChemComm 47, 1100–1105 (2011).
Chen, P., Billett, B. A., Tsukamoto, T. & Dong, G. ‘Cut and sew’ transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Jun, C.-H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Kim, D.-S., Park, W.-J. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev. 117, 8977–9015 (2017).
Zhang, X., Fevre, M., Jones, G. O. & Waymouth, R. M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 118, 839–885 (2018).
Iannone, F. et al. Ionic liquids/ZnO nanoparticles as recyclable catalyst for polycarbonate depolymerization. J. Mol. Catal. A Chem. 426, 107–116 (2017).
Zhang, Q. & Vigier, K. D. O. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 41, 7108–7146 (2012).
Wang, Q. et al. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate) (PET). Green Chem. 17, 2473–2479 (2015).
Raheem, A. B. et al. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: a review. J. Clean. Prod. 225, 1052–1064 (2019).
Al-Sabagh, A. M., Yehia, F. Z., Eshaq, Gh., Rabie, A. M. & ElMetwally, A. E.Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 25, 53–64 (2016).
Chiaie, K. R. D., McMahon, F. R., Williams, E. J., Price, M. J. & Dove, A. P. Dual-catalytic depolymerization of polyethylene terephthalate (PET). Polym. Chem. 11, 1450–1453 (2020). This communication employs Lewis acid–base pairs, in which Zr acid catalysts are used to activate polymer carbonyls, and amine bases are used to increase the reaction rate of the nucleophile, to achieve enhanced depolymerization conversions compared with either of the components in isolation.
Kumar, A. et al. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 142, 14267–14275 (2020). This article reports the hydrogenative depolymerization of nylons, polyamides, and polyurethanes to produce small-molecule products, employing a series of Ru catalysts to generate products, which are then capable of being repolymerized to regenerate polymers with approximately similar molecular weight to the starting materials.
Gausas, L. et al. Catalytic hydrogenation of polyurethanes to base chemicals: from model systems to commercial and end-of-life polyurethane materials. JACS Au. 1, 517–524 (2021). This article reports the use of catalytic hydrogenation of polyurethanes using an Ir catalyst, as well as other metal complexes, to obtain small-molecule products from a range of polyurethane substrates, including commercial-grade materials.
Lo, J. N., Nutt, S. R. & Williams, T. J. Recycling benzoxazine–epoxy composites via catalytic oxidation. ACS Sustain. Chem. Eng. 6, 7227–7231 (2018). This article discusses work in the depolymerization of benzoxazine–epoxy carbon fibre-reinforced composites for recovery of the fibres through oxidation by an Ru catalyst, followed by hydrolysis to cleave the resin and allow for recovery of intact carbon fibres.
Navarro, C. A. et al. Mechanism and catalysis of oxidative degradation of fiber-reinforced epoxy composites. Top. Catal. 61, 704–709 (2018).
Rorrer, N. A. et al. Combining reclaimed PET with bio-based monomers enables plastics upcycling. Joule 3, 1006–1027 (2019).
Kárpáti, L., Fogarassy, F., Kovácsik, D. & Vargha, V. One-pot depolymerization and polycondensation of PET based random oligo- and polyesters. J. Polym. Environ. 27, 2167–2181 (2019).
Fukushima, K. et al. Advanced chemical recycling of poly(ethylene terephthalate) through organocatalytic aminolysis. Polym. Chem. 4, 1610–1616 (2013).
Gardea, F. et al. Hybrid poly(aryl ether sulfone amide)s for advanced thermoplastic composites. Macromol. Chem. Phys. 215, 2260–2267 (2014).
Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 7, 866–880 (2016).
Wei, R. et al. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 3, 867–871 (2020).
Payne, C. M. et al. Fungal cellulases. Chem. Rev. 115, 1308–1448 (2015).
DiCosimo, R., McAuliffe, J., J. Poulose, A. & Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 42, 6437–6474 (2013).
Vaaje‐Kolstad, G., Horn, S. J., Sørlie, M. & Eijsink, V. G. H. The chitinolytic machinery of Serratia marcescens—a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 280, 3028–3049 (2013).
Kari, J., Andersen, M., Borch, K. & Westh, P. An inverse Michaelis–Menten approach for interfacial enzyme kinetics. ACS Catal. 7, 4904–4914 (2017).
Kari, J. et al. Sabatier principle for interfacial (heterogeneous) enzyme catalysis. ACS Catal. 8, 11966–11972 (2018). This study draws an important analogy between interfacial biocatalysis (which is key to enzymatic plastics deconstruction) and the Sabatier principle (which is often invoked in the design of heterogeneous catalysts). This paper applies these concepts to cellulases, but the design approach is probably translatable to plastic-degrading enzymes as well.
Kari, J. et al. A steady-state approach for inhibition of heterogeneous enzyme reactions. Biochem. J. 477, 1971–1982 (2020).
Arnling Bååth, J., Borch, K., Jensen, K., Brask, J. & Westh, P. Comparative biochemistry of four polyester (PET) hydrolases. ChemBioChem 22, 1627–1637 (2021).
Zaks, A. & Klibanov, A. M. Enzymatic catalysis in nonaqueous solvents. J. Biol. Chem. 263, 3194–3201 (1988).
Iyer, P. V. & Ananthanarayan, L. Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem. 43, 1019–1032 (2008).
Moniruzzaman, M., Kamiya, N. & Goto, M. Activation and stabilization of enzymes in ionic liquids. Org. Biomol. Chem. 8, 2887–2899 (2010).
Roth, C. et al. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl. Microbiol. Biotechnol. 98, 7815–7823 (2014).
Silva, C. M. et al. Cutinase—a new tool for biomodification of synthetic fibers. J. Polym. Sci. Pol. Chem. 43, 2448–2450 (2005).
Araújo, R. et al. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J. Biotechnol. 128, 849–857 (2007).
Ribitsch, D. et al. Characterization of a new cutinase from Thermobifida alba for PET-surface hydrolysis. Biocatal. Biotransformation 30, 2–9 (2012).
Sulaiman, S. et al. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 78, 1556–1562 (2012).
Ronkvist, Å. M., Xie, W., Lu, W. & Gross, R. A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42, 5128–5138 (2009).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016).
Han, X. et al. Structural insight into catalytic mechanism of PET hydrolase. Nat. Commun. 8, 2106 (2017).
Joo, S. et al. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat. Commun. 9, 382 (2018).
Austin, H. P. et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl Acad. Sci. USA 115, 4350–4357 (2018).
Palm, G. J. et al. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 10, 1717 (2019).
Knott, B. C. et al. Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc. Natl Acad. Sci. USA 117, 25476–25485 (2020).
Ribitsch, D. et al. Enhanced cutinase-catalyzed hydrolysis of polyethylene terephthalate by covalent fusion to hydrophobins. Appl. Environ. Microbiol. 81, 3586–3592 (2015).
Várnai, A., Siika-aho, M. & Viikari, L. Carbohydrate-binding modules (CBMs) revisited: reduced amount of water counterbalances the need for CBMs. Biotechnol. Biofuels 6, 30 (2013).
Doi, R. H. & Kosugi, A. Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2, 541–551 (2004).
Carvalho, A. L. et al. Cellulosome assembly revealed by the crystal structure of the cohesin–dockerin complex. Proc. Natl Acad. Sci. USA 100, 13809–13814 (2003).
Resch, M. G. et al. Fungal cellulases and complexed cellulosomal enzymes exhibit synergistic mechanisms in cellulose deconstruction. Energy Environ. Sci. 6, 1858–1867 (2013).
Brunecky, R. et al. Revealing nature’s cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342, 1513–1516 (2013).
Tournier, V. et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219 (2020). This work describes a protein structure-guided design of the leaf compost cutinase enzyme for both higher stability and higher activity against PET substrates. The authors scale-up the process and demonstrate conversion of a post-consumer PET waste stream at industrially relevant enzyme loadings.
Liu, B. et al. Protein crystallography and site-direct mutagenesis analysis of the poly(ethylene terephthalate) hydrolase PETase from Ideonella sakaiensis. ChemBioChem 19, 1471–1475 (2018).
Then, J. et al. Ca2+ and Mg2+ binding site engineering increases the degradation of polyethylene terephthalate films by polyester hydrolases from Thermobifida fusca. Biotechnol. J. 10, 592–598 (2015).
Shirke, A. N. et al. Stabilizing leaf and branch compost cutinase (LCC) with glycosylation: mechanism and effect on PET hydrolysis. Biochemistry 57, 1190–1200 (2018).
Taniguchi, I. et al. Biodegradation of PET: current status and application aspects. ACS Catal. 9, 4089–4105 (2019).
Schreiber, L. Transport barriers made of cutin, suberin and associated waxes. Trends Plant. Sci. 15, 546–553 (2010).
Herrero Acero, E. et al. Enzymatic surface hydrolysis of PET: effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 44, 4632–4640 (2011).
Rahikainen, J. L. et al. Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 133, 270–278 (2013).
Vaaje-Kolstad, G. et al. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330, 219–222 (2010).
Deguchi, T., Kitaoka, Y., Kakezawa, M. & Nishida, T. Purification and characterization of a nylon-degrading enzyme. Appl. Environ. Microbiol. 64, 1366–1371 (1998).
Magnin, A., Pollet, E., Phalip, V. & Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 39, 107457 (2020).
Albertsson, A.-C., Andersson, S. O. & Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stabil. 18, 73–87 (1987).
Inderthal, H., Tai, S. L. & Harrison, S. T. L. Non-hydrolyzable plastics—an interdisciplinary look at plastic bio-oxidation. Trends Biotechnol. 39, 12–23 (2020).
Wierckx, N. et al. Plastic waste as a novel substrate for industrial biotechnology. Microb. Biotechnol. 8, 900–903 (2015).
Narancic, T. & O’Connor, K. E. Microbial biotechnology addressing the plastic waste disaster. Microb. Biotechnol. 10, 1232–1235 (2017).
Linger, J. G. et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl Acad. Sci. USA 111, 12013–12018 (2014).
Utomo, R. N. C. et al. Defined microbial mixed culture for utilization of polyurethane monomers. ACS Sustain. Chem. Eng. 8, 17466–17474 (2020).
Kim, H. T. et al. Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustain. Chem. Eng. 7, 19396–19406 (2019).
Ward, P. G., Goff, M., Donner, M., Kaminsky, W. & O’Connor, K. E. A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic. Environ. Sci. Technol. 40, 2433–2437 (2006). This work represents an early effort in the tandem catalytic–biological approach for plastics depolymerization and biological conversion of the intermediate products to a recyclable material. Specifically, this work employed polystyrene pyrolysis and the use of a Pseudomonas putida strain to produce biodegradable polyhydroxyalkanoates.
Tiso, T. et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 66, 167–178 (2021). This paper demonstrates a two-step process concept for PET upcycling using enzymatic deconstruction with a thermophilic cutinase followed by conversion of the breakdown products in Pseudomonas putida to produce multiple products, including polyurethane precursors and biodegradable plastics.
Olson, D. G., McBride, J. E., Joe Shaw, A. & Lynd, L. R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 23, 396–405 (2012).
Lee, S. Y. et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33 (2019).
Nieto-Domínguez, M. & Nikel, P. I. Intersecting xenobiology and neometabolism to bring novel chemistries to life. ChemBioChem 21, 2551–2571 (2020).
Crosby, J. R. et al. Extreme thermophiles as emerging metabolic engineering platforms. Curr. Opin. Biotechnol. 59, 55–64 (2019).
Salvador, M. et al. Microbial genes for a circular and sustainable bio-PET economy. Genes 10, 373 (2019).
Espinosa, M. J. C. et al. Toward biorecycling: isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 11, 404 (2020).
Bhaganna, P. et al. Hydrophobic substances induce water stress in microbial cells. Microb. Biotechnol. 3, 701–716 (2010).
Cregut, M., Bedas, M., Durand, M.-J. & Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 31, 1634–1647 (2013).
Heipieper, H. J., Neumann, G., Cornelissen, S. & Meinhardt, F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl. Microbiol. Biotechnol. 74, 961–973 (2007).
Acknowledgements
Funding for N.A.R., L.D.E., K.P.S., Y.R.-L., and G.T.B. was provided by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office and Bioenergy Technologies Office. This work was performed as part of the Bio-Optimized Technologies to Keep Thermoplastics out of Landfills and the Environment (BOTTLE) Consortium and was supported by the Advanced Manufacturing Office and Bioenergy Technologies Office under contract number DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by the Alliance for Sustainable Energy. The BOTTLE Consortium includes members from the Massachusetts Institute of Technology, funded under contract number DE-AC36-08GO28308 with the National Renewable Energy Laboratory. J.E.M. was supported by Research England through the Expanding Excellence in England (E3) scheme. N.W. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under grant agreements 863922 (MIX-UP) and 887711 (Glaukos). The scientific activities of the Bioeconomy Science Center were financially supported by the Ministry of Culture and Science within the framework of the NRW Strategieprojekt BioSC (number 313/323-400-002 13; PlastiCycle). We thank R. Clare for help with figure preparation. We thank colleagues in our research groups and collaborators for helpful discussions that have influenced many of the perspectives put forward here.
Author information
Authors and Affiliations
Contributions
L.D.E., N.A.R., K.P.S., and G.T.B. wrote the first draft of the manuscript, which was edited and approved by all authors. All authors contributed to the intellectual efforts for the review; specifically, M.O., J.E.M., and N.W. contributed to the biological catalysis component of the review and Y.R.-L. contributed to the chemical catalysis component of the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Pedro Castaño, Amar Mohanty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ellis, L.D., Rorrer, N.A., Sullivan, K.P. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat Catal 4, 539–556 (2021). https://doi.org/10.1038/s41929-021-00648-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00648-4
This article is cited by
-
Combinatorial high throughput methodologies: the potentials in heterogeneous catalysts synthesis, screening and discovery—a review
Bulletin of the National Research Centre (2024)
-
Bio-upcycling of even and uneven medium-chain-length diols and dicarboxylates to polyhydroxyalkanoates using engineered Pseudomonas putida
Microbial Cell Factories (2024)
-
Natural diversity screening, assay development, and characterization of nylon-6 enzymatic depolymerization
Nature Communications (2024)
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Computational redesign of a hydrolase for nearly complete PET depolymerization at industrially relevant high-solids loading
Nature Communications (2024)