Abstract
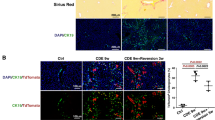
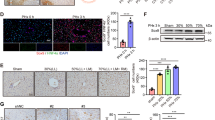
Kupffer cells (KCs), which are liver-resident macrophages, originate from the fetal yolk sac and represent one of the largest macrophage populations in the body. However, the current data on the origin of the cells that restore macrophages during liver injury and regeneration remain controversial. Here, we address the question of whether liver macrophage restoration results from circulating monocyte infiltration or local KC proliferation in regenerating livers after partial hepatectomy (PHx) and uncover the underlying mechanisms. By using several strains of genetically modified mice and performing immunohistochemical analyses, we demonstrated that local KC proliferation mainly contributed to the restoration of liver macrophages after PHx. Peak KC proliferation was impaired in Il6-knockout (KO) mice and restored after the administration of IL-6 protein, whereas KC proliferation was not affected in Il4-KO or Csf2-KO mice. The source of IL-6 was identified using hepatocyte- and myeloid-specific Il6-KO mice and the results revealed that both hepatocytes and myeloid cells contribute to IL-6 production after PHx. Moreover, peak KC proliferation was also impaired in myeloid-specific Il6 receptor-KO mice after PHx, suggesting that IL-6 signaling directly promotes KC proliferation. Studies using several inhibitors to block the IL-6 signaling pathway revealed that sirtuin 1 (SIRT1) contributed to IL-6-mediated KC proliferation in vitro. Genetic deletion of the Sirt1 gene in myeloid cells, including KCs, impaired KC proliferation after PHx. In conclusion, our data suggest that KC repopulation after PHx is mainly driven by local KC proliferation, which is dependent on IL-6 and SIRT1 activation in KCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dong Z, Wei H, Sun R, Tian Z. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4:241–52.
Markose D, Kirkland P, Ramachandran P, Henderson NC. Immune cell regulation of liver regeneration and repair. J Immunol Regen Med. 2018;2:1–10.
Gao B, Jeong W-I, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–36.
Heymann F, Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110.
Merlin S, Bhargava KK, Ranaldo G, Zanolini D, Palestro CJ, Santambrogio L, et al. Kupffer cell transplantation in mice for elucidating monocyte/macrophage biology and for potential in cell or gene therapy. Am J Pathol. 2016;186:539–51.
Nguyen-Lefebvre AT, Horuzsko A. Kupffer cell metabolism and function. J Enzymol Metab. 2015;1:101.
Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45–56.
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21.
Cai J, Zhang X-J, Li H. The role of innate immune cells in nonalcoholic steatohepatitis. Hepatology. 2019;70:1026–37.
Wang H, Mehal W, Nagy LE, Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18:73–91.
Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–6.
Guillot A, Buch C, Jourdan T. Kupffer cell and monocyte-derived macrophage identification by immunofluorescence on formalin-fixed, paraffin-embedded (FFPE) mouse liver sections. In: Aouadi M, Azzimato V (eds). Kupffer cells: methods and protocols. New York, NY: Springer US, 2020, pp 45–53.
Hsieh S-LE, Yang C-Y. CLEC4F, a Kupffer cells specific marker, is critical for presentation of alfa-galactoceromide to NKT cells. J Immunol. 2009;182:78.
Yang C-Y, Chen J-B, Tsai T-F, Tsai Y-C, Tsai C-Y, Liang P-H, et al. CLEC4F is an inducible C-type lectin in F4/80-positive cells and is involved in alpha-galactosylceramide presentation in liver. PLoS ONE. 2013;8:e65070–e65070.
Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91.
Borst K, Frenz T, Spanier J, Tegtmeyer P-K, Chhatbar C, Skerra J, et al. Type I interferon receptor signaling delays Kupffer cell replenishment during acute fulminant viral hepatitis. J Hepatol. 2018;68:682–90.
Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–58.
Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A, et al. Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cell Immunol. 2017;322:74–83.
Lefere S, Degroote H, Van Vlierberghe H, Devisscher L. Unveiling the depletion of Kupffer cells in experimental hepatocarcinogenesis through liver macrophage subtype-specific markers. J Hepatol. 2019;71:631–3.
Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–53.
Nishiyama K, Nakashima H, Ikarashi M, Kinoshita M, Nakashima M, Aosasa S, et al. Mouse CD11b+Kupffer cells recruited from bone marrow accelerate liver regeneration after partial hepatectomy. PLoS ONE. 2015;10:e0136774.
Reid DT, Reyes JL, McDonald BA, Vo T, Reimer RA, Eksteen B. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLoS ONE. 2016;11:e0159524.
Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gélineau A, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53:627–40.
Elchaninov AV, Fatkhudinov TK, Usman NY, Kananykhina EY, Arutyunyan IV, Makarov AV, et al. Dynamics of macrophage populations of the liver after subtotal hepatectomy in rats. BMC Immunol. 2018;19:23.
Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53.
Ichikawa T, Zhang Y-Q, Kogure K, Hasegawa Y, Takagi H, Mori M, et al. Transforming growth factor β and activin tonically inhibit DNA synthesis in the rat liver. Hepatology. 2001;34:918–25.
Weglarz TC, Sandgren EP. Timing of hepatocyte entry into DNA synthesis after partial hepatectomy is cell autonomous. Proc Natl Acad Sci USA. 2000;97:12595–12600.
Melgar-Lesmes P, Edelman ER. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J Hepatol. 2015;63:917–25.
Wen Y, Feng D, Wu H, Liu W, Li H, Wang F, et al. Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of IL-6/Stat3 pathway. Int J Biol Sci. 2015;11:1236–47.
Shan Z, Ju C. Hepatic macrophages in liver injury. Front Immunol 2020;11:322.
Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-κB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Liver Physiol. 2007;292:1570–7.
Meijer C, Wiezer MJ, Diehl AM, Yang S-Q, Schouten HJ, Meijer S, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77.
Böhm F, Köhler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2:294–305.
Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–82.
Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64:1403–15.
He Y, Hwang S, Ahmed YA, Feng D, Li N, Ribeiro M, et al. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol Immunol. 2021;18:18–37.
Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82.
Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, et al. IL-6 trans-signaling controls liver regeneration after partial hepatectomy. Hepatology. 2019;70:2075–91.
Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y et al. Myeloid cell-specific IL-6 signaling promotes miR-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 2020; https://doi.org/10.1002/hep.31658.
Quintana A, Erta M, Ferrer B, Comes G, Giralt M, Hidalgo J. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav Immunol. 2013;27:162–73.
He Y, Feng D, Hwang S, Mackowiak B, Wang X, Xiang X et al. Interleukin-20 exacerbates acute hepatitis and bacterial infection by downregulating Inhibitor of kappa B zeta; target genes in hepatocytes. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.02.004.
Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–21.
Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan H, et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202–15.
Aparicio-Vergara M, Tencerova M, Morgantini C, Barreby E, Aouadi M. Isolation of Kupffer cells and hepatocytes from a single mouse liver BT—alpha-1 antitrypsin deficiency: methods and protocols. In: Borel F, Mueller C (eds). New York, NY: Springer, 2017, pp 161–71.
Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8.
Wynn AA, Miyakawa K, Miyata E, Dranoff G, Takeya M, Takahashi K. Role of granulocyte/macrophage colony-stimulating factor in zymocel-induced hepatic granuloma formation. Am J Pathol. 2001;158:131–45.
Kim AR, Park JI, Oh HT, Kim KM, Hwang J-H, Jeong MG, et al. TAZ stimulates liver regeneration through interleukin-6–induced hepatocyte proliferation and inhibition of cell death after liver injury. FASEB J. 2019;33:5914–23.
Feng D, Dai S, Liu F, Ohtake Y, Zhou Z, Wang H, et al. Cre-inducible human CD59 mediates rapid cell ablation after intermedilysin administration. J Clin Investig. 2016;126:2321–33.
Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321.
Vassiliou I, Lolis E, Nastos C, Tympa A, Theodosopoulos T, Dafnios N, et al. The combined effect of erythropoietin and granulocyte macrophage colony stimulating factor on liver regeneration after major hepatectomy in rats. World J Surg Oncol. 2010;8:57.
Liu H-X, Keane R, Sheng L, Wan, JY Y-. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502–10.
Norris CA, He M, Kang L-I, Ding MQ, Radder JE, Haynes MM, et al. Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS ONE. 2014;9:e96053.
Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz-Leutz E, et al. SIRT1 regulates macrophage self-renewal. EMBO J. 2017;36:2353–72.
Jin J, Iakova P, Jiang Y, Medrano EE, Timchenko NA. The reduction of SIRT1 in livers of old mice leads to impaired body homeostasis and to inhibition of liver proliferation. Hepatology. 2011;54:989–98.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Acknowledgements
Yeni Ait Ahmed was a participant in the NIH Graduate Partnerships Program and a graduate student at the Université Paris-Est-Créteil, France, and is affiliated with the Université Paris-Est-Créteil (UPEC) and the NIH Graduate Partnerships Program. This work was supported by the intramural program of the NIAAA (Bin Gao), the NIH grant R01DK121330, R01DK 122708, R01DK122796 (Cynthia Ju), the Institut Universitaire de France (Fouad Lafdil), and the Ministerio de Economía y Competitividad and European Regional Development Fund RTI2018-101105-B-100 (Juan Hidalgo).
Author information
Authors and Affiliations
Contributions
Y.A.A. designed and performed the surgical procedures and experimental work and wrote the paper. Y.F., R.M.R., Y.H., Y.G., A.G., R.R., and D.F. helped with the PHx surgery, cell isolation, protocol optimization, and other experiments. J.H. and C.J. helped analyze the data and edited the paper. F.L. and B.G. designed and supervised the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ait Ahmed, Y., Fu, Y., Rodrigues, R.M. et al. Kupffer cell restoration after partial hepatectomy is mainly driven by local cell proliferation in IL-6-dependent autocrine and paracrine manners. Cell Mol Immunol 18, 2165–2176 (2021). https://doi.org/10.1038/s41423-021-00731-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-021-00731-7
Keywords
This article is cited by
-
Ten-eleven translocation-2-mediated macrophage activation promotes liver regeneration
Cell Communication and Signaling (2024)