Abstract
Internal N6-methyladenosine (m6A) modifications are among the most abundant modifications of messenger RNA, playing a critical role in diverse biological and pathological processes. However, the functional role and regulatory mechanism of m6A modifications in the immune response to Mycobacterium tuberculosis infection remains unknown. Here, we report that methyltransferase-like 14 (METTL14)-dependent m6A methylation of NAPDH oxidase 2 (Nox2) mRNA was crucial for the host immune defense against M. tuberculosis infection and that M. tuberculosis-secreted antigen EsxB (Rv3874) inhibited METTL14-dependent m6A methylation of Nox2 mRNA. Mechanistically, EsxB interacted with p38 MAP kinase and disrupted the association of TAB1 with p38, thus inhibiting the TAB1-mediated autophosphorylation of p38. Interaction of EsxB with p38 also impeded the binding of p38 with METTL14, thereby inhibiting the p38-mediated phosphorylation of METTL14 at Thr72. Inhibition of p38 by EsxB restrained liquid–liquid phase separation (LLPS) of METTL14 and its subsequent interaction with METTL3, preventing the m6A modification of Nox2 mRNA and its association with the m6A-binding protein IGF2BP1 to destabilize Nox2 mRNA, reduce ROS levels, and increase intracellular survival of M. tuberculosis. Moreover, deletion or mutation of the phosphorylation site on METTL14 impaired the inhibition of ROS level by EsxB and increased bacterial burden or histological damage in the lungs during infection in mice. These findings identify a previously unknown mechanism that M. tuberculosis employs to suppress host immunity, providing insights that may empower the development of effective immunomodulators that target M. tuberculosis.
Similar content being viewed by others
Introduction
M. tuberculosis is an extremely successful intracellular pathogen that causes tuberculosis (TB), which is associated with 10 million active cases and 1.5 million deaths annually1. Upon M. tuberculosis infection, host cells launch a range of cellular innate immune responses; however, M. tuberculosis modulates innate defense mechanisms to promote its intracellular survival2,3,4,5,6,7.
One striking characteristic of M. tuberculosis is its utilization of different type VII secretion systems to secrete numerous proteins across their hydrophobic and impermeable cell walls into the cytoplasm of host macrophages. Several secreted proteins from M. tuberculosis have been shown to either stimulate or inhibit host innate immune responses5. EsxB, which is also named CFP10, is an M. tuberculosis-specific secretory protein encoded by an RD-1 region that is deleted from the vaccine strain Mycobacterium bovis bacille Calmette-Guérin (BCG)8,9,10. It is conventionally considered an M. tuberculosis-specific antigen for diagnosing TB and anti-TB vaccine design because of its ability to stimulate the production of IFN-γ by T lymphocytes11,12. EsxB-induced IFN-γ, TNF-α, and IL-10 are associated with clinical TB13. It has been shown that preincubation of macrophages with recombinant EsxB protein reduces the production of reactive oxygen and nitrogen species8,14,15. However, the exact role and mechanism of EsxB in the pathogenesis of M. tuberculosis infection remain largely unclear.
One important regulatory mechanism of gene expression is the chemical modification of RNA at the post-transcriptional level. N6-methyladenosine (m6A) is the most abundant modification in messenger RNA (mRNA) and non-coding RNA in eukaryotic cells16,17,18,19. The m6A modification of RNA is achieved by the writer complex, composed of such factors as methyltransferase-like 3 (METTL3), METTL14, Wilms’ tumor 1-associated protein (WTAP), and KIAA1429 and removed by the erasers fat mass and obesity-associated protein (FTO) and alkylated DNA repair protein ALKB homolog 5 (ALKBH5)18. The post-transcriptional m6A methylation of adenosines in RNA controls mRNA stability, nuclear processing, translation, RNA–protein interactions, and gene transcription18,20,21,22,23. Increasing evidence has revealed that the m6A methylation of RNA regulates many physiological processes, including development18,19, stem cell differentiation24, DNA damage repair25, and the circadian clock26. Moreover, dysfunctional m6A modification of RNA is associated with various diseases, including tumorigenesis27. However, whether and how m6A mRNA methylation is involved in the pathogenesis of M. tuberculosis remains unclear.
Accumulating evidence indicates that liquid–liquid phase separation (LLPS) is a vital and ubiquitous phenomenon whereby multivalent weak macromolecular interactions drive the transition of some proteins into the formation of biomolecular condensates or droplets that exhibit liquid characteristics28,29,30. These biomolecular condensates or droplets are micron-scale cellular compartments that lack membranous enclosures but selectively enhance protein density, thereby allowing higher rates of biochemical reactions. Recent studies indicate that LLPS plays a vital role in human health and disease31 and that dysregulation of LLPS leads to aberrant condensate and amyloid formation that can contribute to many human diseases, including neurodegeneration and cancer32. However, the role of LLPS in the regulation of immune responses to M. tuberculosis infection remains unclear.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) are enzymes that catalyze the reduction of molecular oxygen to generate superoxide (O2−) and hydrogen peroxide (H2O2) by utilizing NADPH as an electron donor33. Superoxide is converted to H2O2, which is further converted to additional reactive oxygen species (ROS)34,35. There are seven enzymes in the NOX family: NOX1-5 and dual oxidase (DUOX) 1–2. NOX enzymes in humans play important roles in diverse biological functions and vary in expression from tissue to tissue. Importantly, NOX2 is involved in regulating many aspects of innate and adaptive immunity, including regulation of type I interferons, the inflammasome, phagocytosis, antigen processing and presentation, and cell signaling36. Targeting NOX enzymes directly or through scavenging free radicals has been proven to be therapeutically useful for treating autoimmunity and acute lung injury where oxidative stress contributes to pathology36. ROS are produced by NOX2 upon the phagocytosis of pathogens in macrophages to facilitate the killing of M. tuberculosis37. However, whether and how Nox2 is regulated by m6A mRNA methylation has not been reported.
In the present study, we uncover a critical role of m6A mRNA methylation of Nox2 by METTL14 in regulating the anti-mycobacterial immune response, whereby M. tuberculosis-secreted antigen EsxB suppresses the m6A RNA modification of Nox2 to promote its intracellular survival. We provide molecular and genetic evidence demonstrating that EsxB inhibits p38-mediated phosphorylation as well as LLPS of METTL14, thereby reducing the m6A methylation of Nox2 mRNA and the production of ROS and promoting the intracellular survival of M. tuberculosis.
Results
METTL14 regulates the intracellular survival of M. tuberculosis
To investigate whether m6A mRNA methylation is involved in innate immune responses to M. tuberculosis, we transiently transfected mouse peritoneal macrophages with siRNA that targeted RNA methyltransferase METTL14 and analyzed the effects on the intracellular survival of M. tuberculosis in a colony-forming unit (CFU) assay. Silencing of Mettl1438 significantly promoted the ~97% intracellular survival M. tuberculosis in macrophages compared to the control (Supplementary Fig. S1a, b). We next crossed Mettl14flox/flox (mMettl14f/f) mice with LysM-Cre transgenic mice and generated Mettl14flox/flox;LysM-Cre (mMettl14−/−) mice that carried Mettl14 gene deletion in myeloid cells. Using western blotting assay, we confirmed Mettl14 deletion in peritoneal macrophages from mMettl14−/− mice (Supplementary Fig. S1c). Consistently, knockout of Mettl14 in macrophages also increased the intracellular bacteria CFU count at 24 h post-infection (Fig. 1a), while no significant difference in CFU count was observed between mMettl14f/f and mMettl14−/− at 2 h post-infection suggesting that the macrophages isolated from mMettl14−/− mice may have a deficiency in limiting intracellular M. tuberculosis. Mettl14 deficiency had no significant effect on the cell death, indicated by lactate dehydrogenase (LDH) release (Supplementary Fig. S1d), as well as macrophage polarization, as indicated by M1 markers CD86 and MHCII and the M2 marker CD206 (Supplementary Fig. S1e). When infected with M. tuberculosis H37Rv, an equivalent expression of Il1b and Il6 was detected in mMettl14f/f and mMettl14−/− at 4 h post-infection (Supplementary Fig. S1f, g), indicating that Mettl14 may not regulate macrophages inflammatory response. These results suggest that METTL14 may regulate the intracellular survival of M. tuberculosis.
a CFU assay in peritoneal macrophages from control (mMettl14f/f) or Mettl14 conditional knockout (mMettl14−/−) mice infected with H37Rv for 2 h and 24 h (MOI = 5). b Mouse peritoneal macrophages were infected with H37Rv, H37Rv:ΔEsxB, H37Rv(ΔEsxB+GFP), H37Rv(ΔEsxB+EsxB) for 2 h and 24 h, and then subjected to CFU assay (MOI = 5). c–e mMettl14f/f or mMettl14−/− mouse peritoneal macrophages infected with H37Rv, H37RvΔEsxB for 2, 24, 48, and 144 h, and then subjected to CFU assay (MOI = 1, 3, 5). Data in a–e reflect mean ± SEM from three independent biological replicates; two-tailed unpaired Student’s t-test (a–e) was used for statistical analysis.
METTL14 and METLL3 form a heterodimer in which METTL3 is the catalytic component, and METTL14 facilitates the binding of METTL3 to the RNA substrate39. We also used the METTL3-specific inhibitor STM245740 to examine whether the function of METTL14 is m6A-dependent. Inhibition of METTL3 by STM2457 enhanced the survival of M. tuberculosis in mMettl14f/f macrophages to a level similar to that in mMettl14−/− (Supplementary Fig. S1h). Finally, complementation of mMettl14−/− macrophages with wild-type (WT) Mettl14, but not with Mettl14 (R298E), a mutant defective in the binding with METTL341, rescued the intracellular survival of M. tuberculosis (Supplementary Fig. S1h). Altogether, these results suggest that METTL14 is a novel host factor that restricts the intracellular survival of M. tuberculosis through its methyltransferase-related function.
EsxB promotes the intracellular survival of M. tuberculosis via METTL14
To evaluate whether M. tuberculosis-secreted proteins modulate host m6A RNA modification to achieve immune evasion, we infected primary peritoneal macrophages with several M. tuberculosis-secreted protein mutant strains individually, including H37Rv:∆EsxB10, H37Rv:ΔureC42, and H37Rv:ΔnuoG43 and performed a CFU assay. Although the intracellular survival of M. tuberculosis markedly decreased in control macrophages that were infected with all these mutant strains (Fig. 1b; Supplementary Fig. S1i, j), only the lower survival of H37Rv:ΔEsxB was restored in macrophages whose Mettl14 gene was knocked down or knock out (Fig. 1c–e; Supplementary Fig. S1i, k) at 24 h post infection. We further performed CFU assay to examine the intracellular growth of H37Rv and H37Rv:ΔEsxB (MOI = 1, 3, 5) at 2, 24, 48, 72, and 144 h post infection. Under all experimental conditions in Mettl14-intact macrophages, the intracellular survival of the EsxB deletion mutant was significantly lower than that of the WT H37Rv strain, especially up to 6 days post-infection. However, the deletion of Mettl14 eliminated EsxB-mediated intracellular CFU increase (Fig. 1c–e). All these data suggested that EsxB may promote the intracellular survival of M. tuberculosis through METTL14.
EsxB inhibits the stability of Nox2 mRNA
ROS production, apoptosis, autophagy, and lysosomal acidification are crucial cellular antimicrobial events that limit the intracellular survival of mycobacteria44. Treatment with the ROS inhibitor N-acetylcysteine (NAC)45 provided nearly complete abrogation of the enhanced effect of EsxB on the survival of M. tuberculosis in macrophages (Fig. 2a). We used a 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) fluorescent probe to detect ROS levels34 and found that macrophages that were infected with H37Rv:ΔEsxB exhibited much higher ROS levels compared with macrophages that were infected with WT H37Rv (Fig. 2b). Complementation of H37Rv:ΔEsxB with WT EsxB, but not with GFP, inhibited ROS production (Fig. 2b). It has been shown that deletion of EsxB also abolishes the secretion of EsxA (ESAT-6)46,47. Consistently, our data demonstrated that neither EsxB nor EsxA was observed in culture filtrates of H37Rv:ΔEsxB (Supplementary Fig. S2a). It has been shown that EsxA promotes ROS production48,49,50, thus the observed effect of H37Rv:ΔEsxB on ROS production is unlikely to be due to EsxA deficiency. To examine this, we expressed EsxA in immortalized bone marrow-derived macrophages (iBMDM) cells and infected them with H37Rv or H37Rv:ΔEsxB. Expression of EsxA in H37Rv:ΔEsxB-infected macrophages did not significantly change the enhanced effect of ΔEsxB on ROS production (Supplementary Fig. S2b). Together, these results suggest that the observed effects of H37Rv:ΔEsxB on ROS production may primarily be mediated through EsxB deficiency but not EsxA deficiency. It is established that type II interferons play a mandatory role in limiting the survival of M. tuberculosis51. Thus, we analyzed the expression level of Ifng in mMettl14f/f and mMettl14−/− macrophage infected with H37Rv, H37Rv:ΔEsxB, and H37Rv:ΔEsxB+EsxB strains. As shown in Supplementary Fig. S2c, the deletion of EsxB had no significant effects on interferon expression. Thus, EsxB may promote the intracellular survival of M. tuberculosis by inhibiting ROS production. To further investigate the underlying mechanism, we conducted overall pathway analysis in mouse peritoneal macrophages infected with M. tuberculosis H37Rv or H37Rv:∆EsxB by using RNA sequencing (RNA-seq) and subsequent validation via quantitative real-time PCR (qPCR). As shown in Fig. 2c and Supplementary Fig. S2d, e, Nox2 is one of the most significantly inhibited genes by EsxB in H37Rv-infected macrophages. Consistently with this, macrophages infected with H37Rv:ΔEsxB had much higher Nox2 protein levels than macrophages infected with H37Rv, suggesting that EsxB may inhibit Nox2 mRNA levels (Fig. 2d). In addition, tumor necrosis factor gene (Tnf)52,53, chemokine and chemotactic receptor-related genes (C5ar1, Ccl4, and Mcoln2), formyl peptide receptor gene (Fpr1), insulin-like growth factor (Igf1), adhesion molecule (Clmp), and the interferon-gamma inducible gene (Ifi47) were also found to be among the top ten genes that were most significantly inhibited by EsxB, suggesting an inhibitory effect of EsxB on the expression of these pathways.
a, b Mouse peritoneal macrophages pre-treated with DMSO, NAC (ROS inhibitor) and infected with H37Rv or H37Rv(ΔEsxB) (MOI = 5) for 2 and 24 h, and then subjected to CFU assay (MOI = 5). b Changes in the levels of ROS (DCF staining; green) in the macrophages upon H37Rv, H37Rv:ΔEsxB, H37Rv(ΔEsxB+GFP) and H37Rv(ΔEsxB+EsxB) infection for 4 h (MOI = 5). c, d qPCR analysis of Nox2 mRNA level (c) and immunoblot (IB) of Nox2 protein levels (d) from peritoneal macrophages infected with H37Rv, H37Rv:ΔEsxB, H37Rv(ΔEsxB+GFP) and H37Rv(ΔEsxB+EsxB) (MOI = 5) for 4 h. e Peritoneal macrophages from WT or Nox2−/− mice were infected with H37Rv, H37Rv(ΔEsxB) for 4 h, and then the ROS level was detected (MOI = 5). f CFU assay in peritoneal macrophages from WT or Nox2−/− mice infected with H37Rv or H37Rv(ΔEsxB) for 2 h and 24 h, and then subjected to CFU assay (MOI = 5). g Nox2 mRNA stability analysis in macrophages after actinomycin D treatment. Mouse peritoneal macrophages were infected with H37Rv, H37Rv:ΔEsxB, H37Rv(ΔEsxB + GFP), and H37Rv(ΔEsxB + EsxB) (MOI = 5) for 4 h and treated with actinomycin D (5 μg/mL). Cells were harvested at the indicated timepoints. Expression levels were normalized to 0 h and Gapdh was used as a reference gene. Results in d are representative images from one of three independent experiments. All of the results (except d) reflect the mean ± SEM from three independent biological experiments. Two-tailed unpaired Student’s t-test were used in a–c and e–g.
To examine whether EsxB suppresses ROS production via Nox2, we detected ROS production in WT or Nox2−/− peritoneal macrophages infected with H37Rv and H37Rv:ΔEsxB (Supplementary Fig. S2f). As shown in Fig. 2e, deletion of Nox2 nearly eliminated EsxB-mediated inhibition of ROS production. The knockdown or knockout of Nox2 markedly attenuated the enhanced effect of EsxB on the survival of M. tuberculosis in macrophages (Fig. 2f; Supplementary Fig. S2g, h) but had no significant effect on M. tuberculosis-induced cell death (Supplementary Fig. S2i). Altogether, these results suggest that EsxB may suppress ROS production by inhibiting Nox2 mRNA levels, thereby promoting the intracellular survival of M. tuberculosis.
Given that EsxB inhibits Nox2 mRNA levels in M. tuberculosis-infected macrophages, which may result from post-transcriptional effects of mRNA stability, we treated WT H37Rv- and H37Rv:ΔEsxB-infected primary peritoneal macrophages with the transcription inhibitor actinomycin D54, examined Nox2 mRNA levels, and calculated the rate of the half-life of Nox2 mRNA. Both the level and half-life of Nox2 mRNA were substantially higher in H37Rv:ΔEsxB-infected macrophages than in macrophages infected with WT H37Rv (Fig. 2c, g), suggesting that EsxB may inhibit the stability of Nox2 mRNA. To examine whether EsxB specifically inhibits the stability of Nox2 mRNA, we treated H37Rv- or H37Rv:ΔEsxB-infected peritoneal macrophages with actinomycin D, and then analyzed the mRNA levels of key regulators of the ROS- and RNS-related genes, including Nox1-5, Nrf2, Duox1, Xor, Sod1, iNos and Pink1. The data showed that only Nox2 mRNA stability was significantly inhibited by EsxB (Supplementary Fig. S2j). These results suggest that EsxB may specifically inhibit the stability of Nox2 mRNA in M. tuberculosis-infected macrophages.
METTL14 regulates the m6A methylation of Nox2 mRNA
Considering the major effect of m6A RNA methylation on mRNA lifespan55, we sought to determine whether Nox2 RNA is modified by m6A methylation in M. tuberculosis-infected macrophages. To examine the abundance of m6A methylation on Nox2 transcripts, total RNA from uninfected and H37Rv-infected macrophages was subjected to m6A immunoprecipitation and methylated RNA immunoprecipitation sequencing (MeRIP)56. By scoring 24,029 and 24,723 m6A peaks in uninfected and H37Rv-infected macrophages, respectively, higher m6A enrichment on Nox2 RNA was found (Fig. 3a). m6A RNA methylation mostly occurs on conserved RRACH sequence motifs, in which R denotes A or G, and H denotes A, C, or U57. We found several RRACH motifs on the Nox2 sequences (Supplementary Fig. S3a). Using RNA immunoprecipitation (RIP)58, we found that METTL14 bound to Nox2 mRNA in M. tuberculosis-infected primary macrophages (Fig. 3b). We also detected Mettl14 in the pulldown of m6A-modified mRNAs in H37Rv infected peritoneal macrophages (Supplementary Fig. S3b). Moreover, Mettl14 knockdown in primary peritoneal macrophages significantly reduced m6A modification levels of Nox2 mRNA in response to M. tuberculosis infection (Fig. 3c). To examine whether methylation by METTL14 is specific to Nox2 mRNA, we analyzed the mRNA methylation level of several ROS-and RNS-related genes including Nox1-5, Nrf2, Duox1, Xor, Sod1, iNos and Pink1 from H37Rv:ΔEsxB-infected mMettl14f/f or mMettl14−/− peritoneal macrophages. As shown in Supplementary Fig. S3c, methylation by METTL14 was particularly extensive for the mRNA of the Nox2 gene, but not for the other genes. These results suggest that METTL14 may specifically mediate the methylation of Nox2 mRNA. To our knowledge, these data demonstrate for the first time that METTL14 modulates the m6A methylation of Nox2 mRNA during M. tuberculosis infection.
a MeRIP-seq data of Nox2 in macrophages infected with H37Rv for 2 h. b RIP-qPCR for Nox2 mRNA in mMettl14f/f and mMettl14−/− peritoneal macrophages infected with H37Rv for 2 h (MOI = 5) by using anti-Mettl14 antibody. Rabbit IgG served as control. c MeRIP-qPCR analysis of relative m6A Nox2 mRNA in si-Ctrl or si-Mettl14 macrophages infected with H37Rv for 2 h. d Nox2 mRNA stability analysis in si-Ctrl, si-Mettl14 or Igf2bp1-knockdown (si-Igf2bp1) mouse peritoneal macrophages infected with H37Rv for 3 h (MOI = 5) and treated with 5 μg/mL actinomycin D (ActD) for another 4 h. e Nox2 mRNA levels analysis in si-Ctrl, si-Mettl14 or si-Igf2bp1 mouse peritoneal macrophages infected with H37Rv for 3 h (MOI = 5). f qPCR analysis of Nox2 mRNA from mMettl14f/f and mMettl14−/− mouse macrophages infected with H37Rv or H37Rv(ΔEsxB) (MOI = 5) for 4 h. g Real-time fluorescence amplification curves and bar plot of the threshold cycle (CT) of qPCR showing SELECT results for detecting m6A3656 in Nox2 mRNA in mMettl14f/f and mMettl14−/− mouse peritoneal macrophages infected with H37Rv and H37Rv:ΔEsxB for 2 h (MOI = 5). Rn is the raw fluorescence for the associated well normalized to the fluorescence of the passive reference dye (ROX). h Changes in the levels of ROS (DCF staining; green) in the mMettl14f/f or mMettl14−/− macrophages infected with H37Rv or H37Rv(ΔEsxB) for 4 h (MOI = 5). Results in b–h reflect the mean ± SEM from three independent biological experiments. Two-tailed unpaired Student’s t-test were used in (b–h).
It is reported that insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) impair mRNA decay by interfering with mRNA targeted by endonucleases59. The silencing of either Mettl14 or Igf2bp1 by siRNA or deletion of Mettl14 resulted in a decrease in the stability of Nox2 mRNA in M. tuberculosis-infected primary macrophages (Fig. 3d; Supplementary Fig. S3d, e). The binding of Nox2 mRNA with IGF2BP1 was also validated by RNA immunoprecipitation sequencing (RIP-seq) and RNA immunoprecipitation quantitative real-time PCR (RIP-qPCR), suggesting that Nox2 is a target mRNA of IGF2BP1 (Supplementary Fig. S3f, g). Consistent with this finding, Mettl14- and Igf2bp1-knockdown by siRNA or deletion Mettl14 led to much lower levels of Nox2 mRNA in macrophages infected with H37Rv M. tuberculosis (Fig. 3e, f; Supplementary Fig. S3h). These results suggest that the m6A methylation pathway may regulate Nox2 mRNAs in a post-transcriptional and IGF2BP1-dependent manner.
IGF2BP1/2/3 are a new family of m6A readers that guard m6A-modified mRNAs from decay60,61,62. To further explore the potential redundant functions of other IGF2BPs in regulating Nox2 mRNA stability in H37Rv-infected macrophages, we detected the Igf2bp1/2/3 expression and found that Igf2bp1 had the most abundant mRNA level in M. tuberculosis H37Rv-infected macrophages (Supplementary Fig. S3i). Moreover, silencing of Igf2bp2 or Igf2bp3 in macrophages had no significant effect on EsxB-mediated reduction of mRNA stability, mRNA content, or protein level of Nox2 (Supplementary Fig. S3j–l). Together, these results suggested that IGF2BP1, not other IGF2BP proteins, may modulate the mRNA stability of Nox2 in M. tuberculosis H37Rv-infected macrophages.
EsxB inhibits the m6A methylation of Nox2 mRNA via METTL14
We next investigated whether EsxB inhibits the m6A methylation of Nox2 mRNA via METTL14. By using MeRIP-qPCR, we found the knockdown of Mettl14 by specific siRNA or deletion of Mettl14 reduced the m6A methylation of Nox2 mRNA in H37Rv:ΔEsxB-infected macrophages to levels comparable to cells infected with H37Rv (Supplementary Fig. S3m, n). We also determined the methylation sites by performing single-base elongation- and ligation-based qPCR amplification (SELECT) analysis for the detection of single m6A locus at single-base resolution63, and found that m6A levels of A3656 in the 3′ UTR of Nox2 were significantly increased upon H37Rv(ΔEsxB) infection, compared to those infected with H37Rv, but this increment was not observed in mMettl14−/− macrophages (Fig. 3g). Deletion of Mettl14 in macrophages abolished the EsxB-mediated Nox2 decrement (Supplementary Fig. S3o). Moreover, the knockout of Mettl14 eliminated the inhibitory effect of EsxB on ROS production in H37Rv-infected macrophages (Fig. 3h). Although EsxB showed little inhibition on mitochondrial ROS, the deletion of Mettl14 did not significantly change EsxB-mediated mitochondrial ROS production (Supplementary Fig. S3p). These results suggest that EsxB may inhibit the METTL14-mediated m6A methylation of Nox2 mRNA, thereby reducing ROS production during M. tuberculosis infection.
LLPS of METTL14 is dependent on Thr72
In the nucleus of M. tuberculosis-infected macrophages, we frequently observed the robust induction of METTL14 condensates (Fig. 4a, b; Supplementary Fig. S4a). To examine whether METTL14 forms these condensates through LLPS in vitro, we purified recombinant GFP-METTL14 protein from Escherichia coli BL21. The addition of 2 μM METTL14 protein induced robust protein droplets that dramatically decreased with the addition of 10% 1,6-hexanediol (1,6-HEX; an inhibitor of LLPS) (Fig. 4c). When fluorescence recovery after photobleaching (FRAP) was performed 10 min after the initiation of phase separation, METTL14 fluorescence did not efficiently recover (Supplementary Fig. S4b, c), suggesting that METTL14 droplets are less dynamic in vitro.
a, b Immunofluorescence staining for METTL14 in mouse peritoneal macrophages infected with H37Rv for 2 h (MOI = 5), Scale bar, 10 μm. Every point in b represents the number of Mettl14 foci in 30 cells. The foci in the nucleus with a diameter over 200 nm were included. c METTL14 forms liquid droplets in vitro. Scale bar, 5 μm. d, e Immunofluorescence staining of METTL14 in iBMDM cells expressing FLAG-Mettl14-WT or FLAG-Mettl14-T72A stimulated with PGN for 2 h. Scale bar, 10 μm. Every point represents the number of Mettl14 foci in 30 cells. The foci in the nucleus with a diameter over 200 nm were included. f LLPS of GFP-Mettl14-WT and GFP-Mettl14-T72D. g IB and immunoprecipitation (IP) in mMettl14−/− macrophages transfected with plasmids encoding HA-tagged WT or T72A mutants of Mettl14. h MeRIP-qPCR analysis of relative m6A Nox2 mRNA in mMettl14f/f or mMettl14−/− macrophages transfected with vector (-) or plasmids encoding FLAG tagged WT or T72A mutants of Mettl14 and infected with H37Rv or H37Rv(ΔEsxB) for 2 h (MOI = 5). Rabbit IgG served as control. i Nox2 mRNA stability analysis in mMettl14f/f or mMettl14−/− macrophages transfected with vector or plasmids encoding FLAG-tagged WT or T72A mutants of Mettl14 after actinomycin D treatment. Macrophages were infected with H37Rv(ΔEsxB) for 4 h (MOI = 5) before being treated with actinomycin D (5 μg/mL). Real-time fluorescence amplification curves and bar plot of the threshold cycle (CT) of qPCR showing SELECT results for detecting m6A3656 in Nox2 mRNA (j) and Nox2 mRNA stability (k) in peritoneal macrophages from WT or Mettl14T72A mice infected with H37Rv(ΔEsxB) for 2 or 4 h (MOI = 5). Rn is the raw fluorescence for the associated well normalized to the fluorescence of the passive reference dye (ROX). Changes in the Nox2 protein levels in mMettl14f/f and mMettl14−/− macrophages transfected with vector (-) or plasmids encoding FLAG-tagged WT or T72A mutants of Mettl14 (l), or in WT and Mettl14T72A macrophages (m) infected with H37Rv(ΔEsxB) for 0, 4, 8 h (MOI = 5). n Changes in the levels of ROS (DCF staining; green) in mMettl14f/f or mMettl14−/− macrophages transfected with vector (-) or plasmids encoding FLAG-tagged WT or T72A mutants of Mettl14 infected with H37Rv for 4 h (MOI = 5). Results in g, l and m are representative images from one of three independent experiments. Results in c and f were repeated twice independently. Results in b, e, h–k, n reflect the mean ± SEM from three independent biological experiments. Two-tailed unpaired Student’s t-test were used in b, e, h, j, n.
To examine whether post-translational modifications of intrinsically disordered regions (IDRs) regulate METTL14 phase separation, we analyzed the sequence of the IDR in METTL14 and found that Thr72 is a conserved site across various species (Supplementary Fig. S4d). The substitution of threonine at 72 to alanine (T72A) abolished the peptidoglycan (a major component of the M. tuberculosis cell wall)-induced formation of METTL14 condensates in the nucleus of macrophages that overexpressed FLAG-METTL14 or FLAG-METTL14T72A (Fig. 4d, e). Moreover, T72D mutant of METTL14, the phosphorylated form, formed more droplets in vitro (Fig. 4f). These data suggest that M. tuberculosis infection may induce the Thr72-dependent LLPS of METTL14 in macrophages.
We next investigated whether the LLPS of METTL14 regulates METTL14–METTL3 complex formation and, subsequently m6A RNA methylation. M. tuberculosis infection dramatically induced the formation of the METTL14–METTL3 complex in macrophages that were transfected with Mettl14 but not the LLPS-defective T72A mutant of Mettl14 (Fig. 4g). The transfection of mMettl14−/− peritoneal macrophages with WT Mettl14 rather than the T72A mutant restored the m6A methylation and stability of Nox2 mRNA (Fig. 4h, i). In Mettl14T72A/T72A knock-in mice (Supplementary Fig. S4e), we found that Mettl14T72A/T72A macrophages infected with M. tuberculosis H37Rv exhibited significantly less Nox2 mRNA m6A methylation and reduced mRNA stability (Fig. 4j, k; Supplementary Fig. S4f). Consistently, we observed much lower Nox2 protein level in Mettl14T72A/T72A knock-in macrophages or mMettl14−/− macrophages complemented with Mettl14(T72A) (Fig. 4l, m). Moreover, the decrease in ROS production in mMettl14−/− peritoneal macrophages recovered when complemented with WT Mettl14 but not the T72A mutant (Fig. 4n). These results suggest that Thr72-dependent LLPS is essential for METTL14 to modulate the m6A methylation of Nox2 mRNA.
EsxB inhibits LLPS of METTL14
Given that EsxB promotes the intracellular survival of M. tuberculosis via METTL14, we next investigated whether EsxB affects the LLPS of METTL14. We found that the deletion of EsxB markedly increased the formation of METTL14 condensates in M. tuberculosis-infected macrophages that reversed upon treatment with 1,6-HEX (Fig. 5a, b; Supplementary Fig. S5a–c). However, H37Rv and H37Rv△EsxB equally induced cytosolic foci of YTHDF2, another m6A binding protein that also undergoes LLPS in vitro and in vivo64(Supplementary Fig. S5d, e). These results suggest that EsxB inhibits LLPS specifically for METTL14. The complementation of mMettl14−/− macrophages with the Mettl14T72A mutant but not with Mettl14 eliminated the inhibitory effect of EsxB on ROS production (Fig. 5c). EsxB did not enhance the intracellular survival of mycobacteria in mMettl14−/− macrophages that were complemented with the Mettl14T72A mutant that was seen in cells with Mettl14 (Fig. 5d). Consistent with these findings, Mettl14T72A/T72A macrophages infected with M. tuberculosis H37Rv:ΔEsxB did not exhibit the increase of ROS production (Fig. 5c) or decrease of bacteria survival (Fig. 5d) as in WT macrophages. Altogether, these results suggest that EsxB may inhibit the Thr72-dependent LLPS of METTL14, thereby suppressing ROS production to facilitate the intracellular survival of mycobacteria.
a, b Immunofluorescence staining for Mettl14 in mouse peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB) and H37Rv(ΔEsxB + EsxB) for 2 h (MOI = 5). Scale bar, 10 μm. Every point in b represents the number of Mettl14 foci in 30 cells. c, d The foci in the nucleus with a diameter over 200 nm were included. ROS assay (c) and bacterial survival ratio (CFU at 24 h/CFU at 2 h) (d) in mMettl14f/f or mMettl14−/− macrophages transfected with vector (-) or plasmids encoding FLAG-tagged WT or T72A mutants of Mettl14, and WT or Mettl14T72A macrophages infected with H37Rv or H37Rv(ΔEsxB) (MOI = 5) for 4 h in (c); 2 h and 24 h in (d). Results in (a) were repeated twice independently. Results in c, d reflect the mean ± SEM from at least three independent biological experiments. Two-tailed unpaired Student’s t-test were used.
EsxB inhibits p38-mediated phosphorylation of METTL14
We next investigated the possible upstream signaling that regulates the LLPS of METTL14. siRNA knockdown of p38α (p38) markedly reduced the peptidoglycan (PGN)-induced formation of METTL14 condensates (Supplementary Fig. S6a–c). Consistently, p38-deficient macrophages stimulated with PGN showed no increase in METTL14 LLPS, suggesting that M. tuberculosis infection may trigger the LLPS of METTL14 via p38 (Fig. 6a, b; Supplementary Fig. S6c–e). Co-immunoprecipitation (Co-IP) analysis showed that endogenous p38 interacted with METTL14 (Fig. 6c). In an in vitro GST precipitation assay, purified recombinant GST-p38 was pulled down together with GFP-METTL14, indicating a direct interaction between p38 and METTL14 (Fig. 6d). Upon infection with M. tuberculosis, endogenous p38 was rapidly recruited to METTL14 in primary peritoneal macrophages (Fig. 6e, f), indicating a stimulus-dependent interaction between p38 and METTL14. Altogether, these results suggest that p38 may interact with METTL14 to promote the LLPS of METTL14.
a, b Immunofluorescence staining for Mettl14 in peritoneal macrophages from p38αfloxp/floxp (p38αf/f) or p38αfloxp/floxp; LysM-Cre (p38α−/−) mice stimulated with or without PGN for 2 h. Scale bar, 10 μm. Every point in b represents the number of Mettl14 foci in 30 cells. c Endogenous interaction of Mettl14 and p38. d Interaction of GST-p38 with GFP-METTL14 in vitro. e, f Endogenous interaction of Mettl14 and p38 in peritoneal macrophages infected with H37Rv for indicated times (MOI = 5). Relative gray intensity of p38 was shown in (f). g In vitro kinase assay of purified recombinant GST-p38 (active) with GFP-Mettl14-WT or GFP-Mettl14-T72A. h Mettl14-WT and Mettl14-T72A are subjected to in vitro kinase assay and then performed in vitro LLPS assay to test the ability of droplet formation. i IB and IP of mouse peritoneal macrophages infected with H37Rv or H37Rv(ΔEsxB) for indicated times (MOI = 5). j IB and IP of HEK293T cells transfected with plasmids encoding FLAG-EsxB for 24 h and stimulated with TNF-α for indicated times. k–n Real-time fluorescence amplification curves and bar plot of the threshold cycle (CT) of qPCR showing SELECT results for detecting m6A3656 in Nox2 mRNA (k), qPCR analysis of Nox2 mRNA (l), Nox2 protein levels (m) and changes in the levels of ROS (DCF staining; green) (n) in peritoneal macrophages from p38αf/f or p38α−/− mice infected with H37Rv or H37Rv(ΔEsxB) for 2 h in k or 4–8 h in (l–n) (MOI = 5). Rn is the raw fluorescence for the associated well normalized to the fluorescence of the passive reference dye (ROX). o IB analysis of protein levels from mouse macrophages infected with H37Rv or H37Rv(ΔEsxB) (MOI = 5) for indicated times. p IB analysis of iBMDM overexpression with Flag-p38 together with vector (-) or TAB1 or MKK6(E) or EsxB. q In vitro kinase assay of purified recombinant 6× His-p38 with TAB1 and EsxB. r IB analysis of mouse peritoneal macrophages treated with DMSO or SB203580 (10 μM) and infected with H37Rv or H37Rv(ΔEsxB) (MOI = 5) for indicated times. s IB and IP of mouse peritoneal macrophages infected with H37Rv or H37Rv(ΔEsxB) for indicated times (MOI = 5). All of the immunoblot data are representative images from one of three independent experiments. Results in h were repeated twice independently. Results in b, f, k, l, n reflect the mean ± SEM from three independent biological experiments. Two-tailed unpaired Student’s t-test were used.
Given that the LLPS of METTL14 depends on its Thr72 site and that p38 interacts with METTL14, we next investigated whether p38 phosphorylates METTL14 at Thr72. To further study the phosphorylation of METTL14 by p38, we generated a mouse monoclonal p-METTL14 antibody that was specific to the phosphorylation of the METTL14 Thr72 site (Supplementary Fig. S6f). In an in vitro kinase assay, the incubation of purified recombinant METTL14 with p38 led to the robust phosphorylation of METTL14 at Thr72, and the mutation of Thr72 markedly reduced the phosphorylation of METTL14 (Fig. 6g). Moreover, when incubated with p38 in vitro, we only observed phosphorylation and LLPS of WT METTL14 rather than T72A mutation (Fig. 6h; Supplementary Fig. S6g). These results suggest that p38 may mediate LLPS of METTL14 via phosphorylation of METTL14 at Thr72.
M. tuberculosis infection induced the phosphorylation of METTL14 at Thr72, indicated by the immunoblot analysis with the phosphorylation-specific antibody (Fig. 6i). Macrophages that were infected with the H37Rv:ΔEsxB strain exhibited much higher METTL14 phosphorylation compared with cells that were infected with WT H37Rv strains (Fig. 6i), suggesting that EsxB may inhibit the phosphorylation of METTL14. Given that p38 phosphorylates METTL14 at Thr72, we supposed that EsxB may target p38 or its upstream signal to inhibit the phosphorylation of METTL14. M. tuberculosis infection activates p38 via transforming growth factor-β (TGFβ)-activated kinase 1 (TAK1) in the Toll-like receptor (TLR) signaling pathway65. We found that EsxB interacted with p38, rather than TAK1 both in EsxB-expressing HEK293T cells or H37Rv-infected macrophages (Supplementary Fig. S6h, i). In HEK293T cells that overexpressed EsxB, p38 bound less with METTL14 (Fig. 6j). The deletion of EsxB from H37Rv enhanced the interaction between p38 and METTL14 in macrophages that were infected with H37Rv (Fig. 6i). In contrast, expression of EsxA in H37Rv:ΔEsxB-infected macrophages did not significantly change the inhibitory effect of EsxB on the interaction of p38 with METTL14 (Supplementary Fig. S6j). These data suggested that EsxB may interact with p38 and impede the interaction of p38 with METTL14, thus inhibiting the p38-mediated phosphorylation of METTL14.
It has been shown that p38 also directly interacts with and activates the AP-1 complex components ATF-2 and TCF (ELK4) to promote gene expression66,67. To further examine whether the inhibition of EsxB is specific for the action of p38 on METTL4, we examined the endogenous interaction between p38 with METTL14, ATF-2, and ELK4 in H37Rv- or H37Rv:ΔEsxB-infected peritoneal macrophages. The data showed that EsxB did not inhibit the interaction between p38 with ATF-2 and ELK4, suggesting that EsxB may specifically inhibit p38-mediated phosphorylation of METTL14 (Supplementary Fig. S6k). Moreover, the deletion of p38 eliminated the inhibitory effect of EsxB on the m6A modification of Nox2 mRNA in H37Rv-infected macrophages (Fig. 6k; Supplementary Fig. S6l). EsxB reduced Nox2 mRNA levels, protein level, and ROS production in WT or control macrophages but not in p38 inhibitor-treated or p38−/− macrophages (Fig. 6l–n; Supplementary Fig. S6m, n). These results suggest that EsxB may disrupt the interaction between METTL14 and p38, thereby inhibiting the p38-mediated phosphorylation of METTL14.
We found that EsxB markedly inhibited the phosphorylation of p38 in M. tuberculosis H37Rv-infected macrophages (Fig. 6o). Phosphorylation of p38 is regulated by its upstream Mitogen-activated protein kinase 3/6 (MKK3/6)-dependent phosphorylation of TGY residues on the active loop of p38 or TAB1-enhanced autophosphorylation of p3866,68,69,70,71. We found that EsxB inhibited the enhanced phosphorylation of p38 by TAB1 in HEK293T cells or in an in vitro kinase assay (Fig. 6p, q), but not by a dominant active MKK6 (MKK6(E)) mutant69 in HEK293T cells (Fig. 6p). Moreover, treatment with SB203580, a specific inhibitor of p38 autophosphorylation69,72, eliminated the inhibitory effect of EsxB on the p38 phosphorylation in M. tuberculosis H37Rv-infected macrophages (Fig. 6r). Finally, deletion of EsxB markedly increased the endogenous association of p38 with TAB1, but not with MKK3/6, in M. tuberculosis H37Rv-infected macrophages (Fig. 6s). Together, these results suggest that EsxB may disrupt the interaction of p38 with TAB1, thus inhibiting the TAB1-mediated autophosphorylation of p38.
EsxB inhibits p38-mediated phosphorylation of METTL14 via Ser22
It has been shown that the deletion of EsxB also abolishes EsxA secretion46,47 (Supplementary Fig. S2a). To further define the functional role of EsxB that is unrelated with its impact on EsxA, we attempted to identify the EsxB sites that do not affect EsxA secretion but are crucial for the inhibitory effect of EsxB on p38-mediated METTL14 phosphorylation. Based on the structure analysis of EsxB, we mutated seven EsxB sites that are predicted to be crucial for the function of EsxB73. Stable production of the EsxB (M4A), EsxB (S22A), EsxB (Q28A), EsxB (Q52A), EsxB (S73A), EsxB (E88A), and EsxB (M98A) mutant protein was detectable in H37Rv:ΔEsxB cell lysates, consistent with the dispensability of these residues for the production of EsxA (Fig. 7a, b). However, only EsxB (S22A) and EsxB (Q52A) mutants kept both EsxB and EsxA protein secretion (Fig. 7a, b). We further examined the function of EsxB (S22A) and EsxB (Q52A) mutants on the phosphorylation of the Mettl14 Thr72 site in M. tuberculosis-infected macrophages and found that H37Rv:ΔEsxB complemented with EsxB or EsxB (Q52A), but not with EsxB (S22A) restored the inhibition of Mettl14 Thr72 phosphorylation by M. tuberculosis infection (Fig. 7c). Moreover, Ser22 was found to be essential for EsxB to inhibit the interaction of p38 with TAB1, and subsequently TAB1-mediated autophosphorylation of p38 in M. tuberculosis-infected macrophages (Fig. 7c, d). Thus, our results suggest that EsxB may inhibit p38-mediated phosphorylation of METTL14 via Ser22.
a, b IB of EsxA or EsxB in pellets and supernatant from H37Rv, H37RvΔEsxB, or H37Rv(ΔEsxB + EsxBWT/mutants) strains. c IB of mouse peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB) or H37Rv(ΔEsxB + EsxBWT/S22A) for indicated times (MOI = 5). d IB and IP of mouse peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB)or H37Rv(ΔEsxB + EsxBWT/S22A) for indicated times (MOI = 5). e MeRIP-qPCR analysis of relative m6A level of Nox2 mRNA in peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB) or H37Rv (ΔEsxB + EsxBWT/S22A) for 2 h. f qPCR analysis of Nox2 mRNA in mouse peritoneal macrophages following H37Rv, H37Rv(ΔEsxB) or H37Rv (ΔEsxB + EsxBWT/S22A) infection for 4 h (MOI = 5). g IB of mouse peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB) or H37Rv (ΔEsxB + EsxBWT/S22A) for indicated times (MOI = 5). h Changes in the levels of ROS (DCF staining; green) in mouse peritoneal macrophages following H37Rv, H37Rv(ΔEsxB) or H37Rv (ΔEsxB + EsxBWT/S22A) infection for 4 h (MOI = 5). i Peritoneal macrophages infected with H37Rv, H37Rv(ΔEsxB) or H37Rv (ΔEsxB + EsxBWT/S22A) for 2 h and 24 h, and then subjected to CFU assay (MOI = 5). Results in a–d, g are representative images from one of three independent experiments. All of the bar graphs in this figure reflect the mean ± SEM from three independent biological experiments. Two-tailed unpaired Student’s t-test were used.
Consistently, H37Rv:ΔEsxB or H37Rv:ΔEsxB+S22A mutant strains induced much higher m6A methylation (Fig. 7e), mRNA (Fig. 7f) and protein amount (Fig. 7g) of Nox2 in M. tuberculosis-infected macrophages. H37Rv:ΔEsxB- or H37Rv:ΔEsxB + S22A mutant-infected macrophages exhibited higher ROS production (Fig. 7h) and lower bacteria survival (Fig. 7i). Thus, our data suggest that the inhibitory effect of ΔEsxB on the ROS production may require its interaction with p38 and TAB1, not through its interruption on the expression and secretion of ESAT-6.
EsxB inhibits host anti-TB immunity via phosphorylation of METTL14
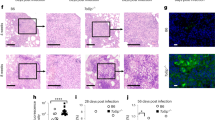
We next investigated the role of EsxB in Mettl14-dependent macrophage immunity during M. tuberculosis infection in mMettl14f/f and mMettl14−/− mice. Both male and female mMettl14−/− mice exhibited normal growth rate and physiology relative to mMettl14f/f counterparts, which is consistent with previous reports74. Notably, for Mettl14T72A/T72A mice, at 8 weeks after infection, nearly 50% of mice died, but all the WT mice survived (Fig. 8a), suggesting that phosphorylation of METTL14 on T72 may promote the anti-TB immunity. Compared with mMettl14f/f mice, mMettl14−/− mice infected with M. tuberculosis exhibited a significant increase in bacterial burden (increased ~1.45 fold in log10 at day 30; increased ~2.23 fold in log10 at day 60) and histological damage in the lungs, supporting the essential role of METTL14 in host anti-TB immunity on controlling M. tuberculosis bacterial loads in vivo (Fig. 8b–e). In mMettl14f/f mice, H37Rv:ΔEsxB markedly reduced M. tuberculosis-induced inflammatory infiltration and bacterial burden in the lungs. However, H37Rv and H37Rv:ΔEsxB caused comparable histopathological changes and bacterial burden in mMettl14−/− mice (Fig. 8b–e). Our results suggest that EsxB is required by M. tuberculosis to evade host Mettl14-mediated immune responses.
a Kaplan–Meier survival curves of mice infected with H37Rv for 8 weeks. Shown is the combined survival from two independent experiments (n = 18 mice per group). b–e 6–8-week-old female mMettl14f/f or mMettl14−/− mice were aerosol-infected with H37Rv (~ 200 CFUs per mouse). CFU of bacterial load at 1, 30 and 60 days post-infection (b); lung sections acid-fast staining (c), haematoxylin and eosin staining (d), and histology score (e). Scale bar, 1 mm. f–i 6–8-week-old female WT or Mettl14T72A/T72A mice infected with H37Rv for 1, 30 and 60 days (~200 CFUs per mouse). We assayed: CFU of bacterial load at 1, 30 and 60 days post-infection (f); lung sections acid-fast staining (g), H&E staining (h), and histology score (i). Scale bar, 1 mm. Data in b and f show cumulative data from three independent experiments (mean ± SEM of n = 18) and a two-sided Mann–Whitney-U-test (b, f).
To further examine the functional role of Mettl14 Thr72 in the EsxB-mediated pathogenesis of M. tuberculosis infection in vivo, we challenged WT and Mettl14T72A/T72A knock-in mice with H37Rv and H37Rv:ΔEsxB. As shown in Fig. 8f–i, Mettl14T72A/T72A mice infected with M. tuberculosis exhibited a substantial increase in histological damage in the lungs than did their WT counterparts. Moreover, Mettl14T72A/T72A mice displayed a significantly increased bacteria load in lung tissues from 30 and 60 days post-infection (increased ~1.3 fold in log10 at day 30; increased ~2.0 fold in log10 at day 60) (Fig. 8f–i). These results provided direct evidence that METTL14 Thr72 is crucial for host anti-TB immunity in vivo. Moreover, the mutation of Mettl14 on Thr72 in mice abolished the differences in histopathological changes and bacterial burden in the lungs of mice infected with H37Rv and H37Rv:ΔEsxB (Fig. 8f–i). Together, these data demonstrate that EsxB is required for the pathogenesis of M. tuberculosis via inhibiting phosphorylation of METTL14-mediated anti-TB immunity.
METTL14 phosphorylation clinically associates with Nox2 mRNA level
Given that M. tuberculosis-secreted protein EsxB inhibits the p38 phosphorylation-dependent LLPS of METTL14, thereby reducing the m6A RNA methylation and mRNA stability of Nox2, which reduces ROS levels and increases intracellular survival of M. tuberculosis (Fig. 8a), we next investigated whether this regulatory mechanism or target is clinically associated with human tuberculosis caused by M. tuberculosis. We collected bronchoalveolar lavage fluid (BALF) cells from PTB (primary TB) or TB-negative patients and analyzed METTL14 T72 phosphorylation, Nox2 mRNA, and ROS production. As shown in Supplementary Fig. S7a–c, BALF cells from PTB patients exhibited higher levels of METTL14 T72 phosphorylation, Nox2 mRNA expression, and ROS production compared to TB-negative patients. These data suggest that METTL14 phosphorylation may be clinically associated with Nox2 mRNA level and ROS production in TB patients.
Discussion
The establishment of TB infection and disease development are largely determined by the interactions between host immune defense and immune evasion of M. tuberculosis2,3,4,5,6,7. m6A is the most abundant modification in mRNA and non-coding RNA in eukaryotic cells16,17,18,19. Our findings identify the mycobacterial secreted antigen EsxB as a previously unrecognized essential factor for the intracellular survival of M. tuberculosis through inhibiting METTL14-mediated m6A methylation of Nox2 mRNA (Supplementary Fig. S7d). We found that M. tuberculosis infection leads to p38-mediated phosphorylation and subsequent LLPS of the m6A RNA “writer” METTL14, thereby enhancing the m6A methylation and stability of Nox2 mRNA. However, the mycobacterial secreted protein EsxB inhibits TAB1-mediated autophosphorylation of p38 and downstream p38-mediated phosphorylation as well as LLPS of METTL14, thus reducing the m6A methylation Nox2 mRNA and the production of ROS to promote the intracellular survival of M. tuberculosis. Thus, our findings not only indicate that m6A RNA modification plays a crucial role in the host defense against M. tuberculosis infection, but also reveal that EsxB appears to be a novel virulence factor that allows M. tuberculosis to employ an unexpected mechanism to evade host innate immunity. This mechanism depends on the reduced m6A mRNA modification of anti-mycobacterial gene transcripts by a mycobacterial secreted antigen.
Although m6A mRNA modification modulates epi-transcriptional regulation of gene expression, participating in multiple physiological and pathological processes17,19,75,76,77, whether m6A modifications regulate the immune responses against M. tuberculosis infection remains unknown. Our present study demonstrated that METTL14 is essential for limiting the intracellular growth of M. tuberculosis in macrophages. During M. tuberculosis infection, METTL14 promoted the m6A methylation of Nox2 mRNA, which binds with IGF2BPs to increase the stability of Nox2 mRNA in M. tuberculosis-infected primary macrophages. Increased production of NOX2 is responsible for the generation of ROS to limit the intracellular survival of mycobacteria44. Furthermore, the deletion of Mettl14 dramatically impaired the anti-TB immunity as indicated by increased bacteria burden and pathological damage in the lung of M. tuberculosis-infected mice. Therefore, our results identified a novel role for m6A modification in innate immunity to M. tuberculosis infection, suggesting that m6A modification may be a potential target for the anti-TB drug or vaccine design.
Biomolecular condensates or droplets formed by LLPS are increasingly recognized as an important cellular phenomenon that is crucial for the regulation of human diseases28,29,30. It has been shown that the cytosolic m6A-binding proteins YTHDF1, YTHDF2, and YTHDF3 undergo LLPS, which can be further enhanced by multiple m6A modifications on RNA64,78,79. A more recent study indicated that the S-adenosylmethionine (SAM)-dependent LLPS of METTL3 dynamically regulates the assembly of the METTL3/METTL14/WTAP writer complex80. We found that M. tuberculosis infection induced the formation of METTL14 condensates through LLPS in macrophages. The formation of condensates through phase separation is often associated with low-complexity IDRs within RNA-binding proteins81,82,83. Moreover, phosphorylation within these disordered regions can control condensate assembly and disassembly28,84,85. Our present study demonstrated that LLPS of METTL14 depends on the phosphorylation of Thr72, a conserved site in the IDR of METTL14, by the p38 MAP kinase. A previous study identified S399 as one of the phosphorylation sites of METTL14, but no function of this site was specified86,87. Our findings demonstrate that phosphorylation of the Thr72 site by p38 is the first identified functional site required for METTL3–METTL14 complex formation and methylation activity, but further study is needed to clarify the functional consequences of p38-mediated phosphorylation of METTL14 on Thr72 by generating Mettl14T72A knock-in mice. Future research will extend to the identification and characterization of the phosphorylation-dependent LLPS of m6A RNA modification regulators in other physiological and pathological processes.
M. tuberculosis is an extremely successful intracellular pathogen that can evade innate immune clearance to establish persistent infections inside macrophages2,3,4,5,6,7. It has been shown that p38 MAPK signaling pathways play critical roles in the M. tuberculosis-induced production of pro-inflammatory cytokines. Furthermore, p38 MAPK phosphorylation is related to inhibition of M. tuberculosis growth in macrophages. p38 MAPK is also associated with Th1 cell activation and differentiation, which is critical for protection against M. tuberculosis. Despite its crucial role in immune responses to M. tuberculosis, the mechanisms underlying the modulation of p38 MAP kinase remain unknown. p38 MAP kinase is activated by its upstream MKK3/6 or TAB166,68,69,70,71. TAB1 interacts with p38 and promotes its autophosphorylation of p3866,68,69,70,71. Our data demonstrate that EsxB disrupts the interaction of p38 with TAB1, thus inhibiting the TAB1-mediated autophosphorylation of p38. Furthermore, our results showed that EsxB specifically inhibits the p38-mediated phosphorylation of METTL4 on T72A to impair ROS production and the clearance of M. tuberculosis. To the best of our knowledge, our study is the first to show the mechanism underlying the modulation of p38 signaling by M. tuberculosis to benefit its intracellular survival. However, given the numerous upstream regulators and downstream substrates of p38 MAPK relevant to the macrophage’s interaction with M. tuberculosis, more studies are needed to explore the regulation of p38 MAP kinase signaling during M. tuberculosis infection.
We found that deletion of EsxB from M. tuberculosis H37Rv enhanced the production of ROS in macrophages, which is consistent with previous reports that the addition of recombinant EsxB protein reduces the production of ROS and RNS in macrophages14,15. Although deletion of EsxB abolishes the secretion of EsxB and EsxA in M. tuberculosis, EsxA and EsxB have completely opposite functions in the regulation of ROS production that EsxA promotes48,49,50, while EsxB inhibits ROS production. In addition, expression of EsxA in iBMDM cells did not significantly change the enhanced effect of ΔEsxB on ROS production indicating that the observed effects of H37Rv:ΔEsxB on macrophages ROS production may primarily be mediated through EsxB deficiency but not EsxA deficiency. To test the specific role of EsxB-inhibited ROS production in H37Rv-infected macrophages, point mutations in EsxB allow substantial levels of secretion of EsxA, while abolished EsxB secretion is underway88,89. Mechanistically, our results showed that EsxB inhibits METTL14-mediated m6A methylation of Nox2 mRNA to promote the intracellular survival of M. tuberculosis. To the best of our knowledge, our study is the first to show the immune evasion of M. tuberculosis at the m6A methylation level. However, we note that the inhibition by WT M. tuberculosis H37Rv on METTL14-mediated function is incomplete. Other METTL14-mediated functions may still contribute to the clearance of intracellular mycobacteria; thus, WT M. tuberculosis grows better when Mettl14 is knocked out. Another report demonstrated that nuoG is an M. tuberculosis virulence factor that inhibits apoptosis via repressing ROS production in the phagosome, though the underlying mechanism remains to be determined43. We found that the intracellular survival of H37RvΔnuoG markedly decreased in mMettl14f/f control macrophages, and the reduced survival of H37RvΔnuoG was not restored in mMettl14−/− macrophages, suggesting that nuoG may repress ROS production to promote the intracellular survival of M. tuberculosis through other mechanisms, rather than METTL14-mediated Nox2 m6A methylation. Whether M. tuberculosis modulates the m6A methylation of other anti-mycobacterial genes to trigger or inhibit the host immune responses will require further investigation.
It has been shown that NOX2 (also named CYBB) polymorphisms are significantly correlated with reduced risk of tuberculosis90 and CYBB missense mutations lead to recurrent BCG disease or recurrent tuberculosis91,92,93. By analyzing the blood gene expression profiles, Deng et al. found that CYBB mRNA is much higher in active pulmonary TB patients (PTB) compared to latent TB patients94. The SNPs of METTL14 rs62328061 GG genotype are significantly increased and the transcription levels of METTL14 were significantly decreased in PTB patients compared to normal controls95. In our study, we observed a clinical association of METTL14 phosphorylation with Nox2 mRNA level and ROS production in PTB patients. Therefore, these findings that METTL14 and related gene expression levels are associated with tuberculosis infection and its severity in tuberculosis patients may provide more insights into the understanding of tuberculosis pathogenesis in humans.
Collectively, our findings provide evidence that m6A RNA modification plays a crucial role in the host immune defense against M. tuberculosis infection. Pathogenic mycobacteria evolved a secreted protein, EsxB, which is widely known as a specific antigen for the diagnosis of TB96, to suppress the m6A RNA modification and mRNA stability of anti-mycobacterial genes, thus highlighting the versatility of host–M. tuberculosis interactions (Supplementary Fig. S7). Our findings provide insights into the functional and regulatory mechanism of m6A RNA modification, raising the possibility of novel anti-tuberculosis treatments that target the EsxB–METTL4 interface.
Materials and methods
Bacterial strains and cells
Bacterial strains are described in Supplementary Table S1. Mycobacterial strains were grown in the following medium: Middlebrook 7H9(BD) broth supplemented with 0.05% Tween-80 (Sigma) and 10% oleic acid–albumin–dextrose–catalase (OADC) or Middlebrook 7H10 agar mixed with 10% OADC. For mycobacterial strain selection, the antibiotic hygromycin or kanamycin was mixed in a concentration of 100 μg/mL. Mycobacteria cultures were grown up to mid-log phase (an optical density at 600 nm of ~0.6). 7H10 solid medium preparation was mixed with 270 mL ddH2O, 5.7 g 7H10, and 1.5 mL glycerol, then, added penicillin (1:1000) and 30 mL OADC to culture tuberculosis from post-infected macrophages or mice.
HEK293T cells (ATCC CRL-3216) were resuspended in DMEM (HyClone) mixed with 10% (v/v) fetal bovine serum (FBS, Gibco) for experiments. Macrophages and THP1 cells (ATCC TIB-202) were cultured in RPMI-1640 medium supplemented with 10% (v/v) FBS. Peritoneal macrophages were obtained from mice (~6 weeks) three days after injection of thioglycollate (BD). All the cells were routinely tested for contamination by mycoplasma.
Plasmids, reagents, and antibodies
Plasmids are described in Supplementary Table S1. The following antibodies and reagents were used for western blotting assay, immunofluorescence, or Co-IP: rabbit anti-GAPDH antibody (Catalog#/Clone: SAB2701826/polyclonal), rabbit anti-GFP antibody (Catalog#/Clone: AB10145/polyclonal), anti-METTL14 antibody (Catalog#/Clone: SAB5700855/ polyclonal), anti-METTL3 (Catalog#/Clone: AV34590) were purchased from Sigma-Aldrich; anti-EsxB antibody (Catalog#/Clone: ab45074) and Anti-ESAT6 antibody (Catalog#/Clone: ab45073) were from Abcam; anti-CYBB-NOX2 antibody (Catalog#/Clone: NBP2-38642/polyclonal) was from Novusbio; rabbit anti-phospho-p38 antibody (Catalog#/Clone: 9215/3D7), anti-p38 MAPK (Catalog#/Clone: 8690/D13E1), were from Cell Signaling Technology, Danvers, MA; anti-YTHDF2 antibody (Catalog#/Clone: 24744-1-AP/polyclonal) was purchased from Proteintech; mouse monoclonal antibody to Mettl14 phosphorylated at Thr72 (p-T72-METTL14) was generated by immunization of rabbits, in collaboration with AbClonal Biotech.
Generation of complementary strain
H37Rv(ΔEsxB + GFP) and H37Rv(ΔEsxB + EsxB) strains were generated as a previous study7. Briefly, the shuttle vector pMV261 (provided by K. Mi, Institute of Microbiology, Beijing, China) was used to complement the strain H37RvΔEsxB with GFP or EsxB. Expression of EsxB in mycobacteria was examined by immunoblot analysis.
Generation of mouse monoclonal p-METTL14 antibody
Mouse monoclonal antibody to Mettl14 phosphorylated at Thr72 (p-T72-METTL14) was generated by immunization of mice, in collaboration with AbClonal Biotech. Briefly, mice were immunized with a mixture of METTL14 Thr72 site by immunizing mice with the METTL14 peptide c(KLH)DEGE-pT-DEDK. c(KLH) is a keyhole limpet hemocyanin that fuses on the peptide through cysteine (“pT” indicates phosphorylated threonine) at a ratio of 1:1. Fusion screens: prepare homologous myeloma cells were mixed with mouse splenocytes in a certain proportion and the profusion agent polyethylene glycol was added. Under the action of polyethylene glycol, various lymphocytes can fuse with myeloma cells to form hybridoma cells. Fluent hybridoma cells were screened using HAT selective medium, laid on 10 plates (96-well plates), and ELISA assays. Subclonal screening: positive hybridoma cells obtained from the original well may be derived from two or more hybridoma cells. The clonal culture of hybridoma cells was carried out by the limited dilution method; each original well of fusion cells was laid on a plate, observed under an inverted microscope, marked wells with only a single clone growth, and the supernatant was taken for ELISA detection, and positive monoclonal cells were screened.
Transfection and reverse transcription-PCR (RT-PCR) analysis
HEK293T cells were transiently transfected with Lipofectamine2000 (cn11668; Invitrogen). For siRNA experiments, the sequences of three siRNAs were designed to silence one specific target gene and peritoneal macrophages were transfected with a mixture of the three siRNAs using siRNA-Mate transfection reagent (G04003, from GenePharma), in accordance with the manufacturer’s instructions. A control siRNA (si-Ctrl: 5′-GGCUCUAGAAAAGCCUAUGCdTdT-3′) was used as a negative control. The three siRNA sequences are described in Supplementary Table S1. RNA preparation and quantitative PCR analysis were performed as previously described97.
MeRIP-seq/qPCR
The RNA was extracted from infected cells, then fragmented to around 300–500 bp (covaris S220). The RNA was mixed with beads binding with NEB m6A antibody (E1610S) (A sample total RNA of 1 μg is taken as an example, which can be divided into two IP repeats). The mixture was divided into two parts at 4 °C for 4 h. The RNA binding on beads was washed with trizol and CHCl3 (1/5 volume), and added to the gel separation EP tube. The RNA was washed twice with 80% EtOH, 13,000 × g, 4 °C for 5 min, then dissolved in 10 μL of water, frozen at –80 °C. The RNA was used to qPCR or built into a library with SMARTer® Stranded Total RNA-Seq Kit.
SELECT detection
At least 3.0 × 107 macrophages were collected after infection and the RNA was extracted from the collected cells. The SELECT detection was analyzed as previously described63. The RNA was mixed with 40 nM Up Primer, 40 nM Down Primer, and 5 μM dTTP (or dNTP) in 17 μL 1× CutSmart buffer (50 mM KAc, 20 mM Tris–HAc, 10 mM MgAc2, 100 μg/mL BSA, pH 7.9). The RNA and primers were annealed by incubating mixture at a temperature gradient: 90 °C for 1 min, 80 °C for 1 min, 70 °C for 1 min, 60 °C for 1 min, 50 °C for 1 min, and then 40 °C for 6 min. Subsequently, a 3 μL mixture containing 0.01 U Bst 2.0 DNA polymerase, 0.5 U SplintR ligase, and 10 nmol ATP was added in the former mixture to the final volume of 20 μL. The final reaction mixture was incubated at 40 °C for 20 min, denatured at 80 °C for 20 min, and kept at 4 °C. Afterwards, the qPCR reaction was performed in Applied Biosystems ViiATM7 Real-Time PCR System (Applied Biosystems, USA) or StepOnePlus™ Real-Time PCR System (Applied Biosystems). The 20 μL qPCR reaction was composed of 2× Hieff qPCR SYBR Green Master Mix (Yeasen) or PowerUp™ SYBR™ Green Master Mix (Applied Biosystems), 200 nM qPCRF primer, 200 nM qPCRR primer, 2 μL of the final reaction mixture and ddH2O. qPCR was run at the following condition: 95 °C, 5 min; (95 °C, 10 s; 60 °C, 35 s) × 40 cycles; 95 °C, 15 s; 60 °C, 1 min; 95 °C, 15 s (collect fluorescence at a ramping rate of 0.05 °C/s); 4 °C, hold. Data were analyzed by QuantStudioTM Real-Time PCR Software v1.3.
RNA-seq and quantitative RT-PCR (RT-qPCR)
Total RNA was extracted using RNA-Quick Purification Kit (ES Science, Guangzhou, China) and cDNA was synthesized using the Prime-Script cDNA synthesis kits (Invitrogen, CA, USA) according to the manufacturer’s instructions. The reverse-transcribed cDNA products were used for qPCR analysis using the SYBR Green PCR kit (Invitrogen, California, USA).
In vitro kinase assay
The recombinant human p38 alpha/MAPK14 protein (Active) (ab268832, abcam) was dissolved in kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, 150 μM ATP, 50 mM NaCl, 0.02% BSA, and 1 mM DTT), then incubated with the recombinant protein METTL14-6 × His, METTL14T72A-6 × His, GFP-Mettl14 or GFP-METTL14T72A (Sangon Biotech) were mixed with at 37 °C for 90 min. Then, the reaction mixture was subjected to LLPS assay or SDS-PAGE.
Measurement of intracellular ROS levels
The intracellular ROS levels of macrophages were measured using a Reactive Oxygen Species Assay Kit (Beyotime Biotechnology, China); 2′,7′-dichlorofluorescein-diacetate (DCFH-DA), which is oxidized to fluorescent dichlorofluorescein (DCF) by intracellular ROS, is its principal component. The cells infected by different H37Rv strains were seeded in 96-well. Following the treatment, the cells were incubated with DCFH-DA for 20 min at 37 °C and then defined using fluorescence microscopy (Olympus) and measured at 488 nm excitation and 525 nm emission by a fluorescence spectrophotometer. Finally, the ROS levels could be quantified.
Measurement of mito ROS
To detect mitochondrial superoxide mitoSOX (ThermoFitablesher) was used. Cells were stained for 10 min in 5 μM mitoSOX in 1× PBS 2% FBS at 37 °C. Image J software was used for image analysis. Mean fluorescence intensity was calculated from images by the Leica SP8 confocal microscopy system.
RNA stability assay
For RNA stability assay, Nox2 mRNA stability analysis in mouse peritoneal macrophages after 5 μg/mL actinomycin D (ActD) treatment. Peritoneal macrophages were transfected with siRNAs targeting Mettl14 or Igf2bp1 or a control sequence. 48 h later, a time course for RNA stability was started by infection, and then the transcription inhibitor (actinomycin D) was added. Cells were collected at the indicated timepoints. Expression levels were normalized to “0 h” and GAPDH was set as the reference gene.
RNA half-life measurements
Nox2 mRNA half-life was analyzed as previously described98. Mouse peritoneal macrophages were infected with different M. tuberculosis strains (MOI = 5) for 4 h and treated with actinomycin D (5 μg/mL). Cells were harvested at the indicated time points and analyzed via RT-PCR. Nox2 mRNA levels were normalized to 0 h and Gapdh was used as a reference gene. Nox2 mRNA half-life is calculated according to the following equation: ln(Ci/C0) = −kti. In this equation k is the degradation rate, Ci is the mRNA value at time i, and ti is the time interval in hours. We first calculated the average degradation rate (ka) from each time point ki. The mRNA half-life t1/2 is ln(2)/ka.
RIP-seq and RIP-qPCR
For RIP-seq, macrophages after infection were collected and then the pellet was resuspended in lysis buffer and rotated for 30 min at 4 °C. After cell lysis, the lysate was harvested by centrifugation at 12,000 × g for 10 min. The supernatant was transferred into a fresh 1.5 mL tube. Protease inhibitors and RNase inhibitors were added to the lysis buffer. About 10% volume of lysate was kept and the RNA was extracted as the input to detect the RNA integrity. The following RIP steps were performed by using an RNA-immunoprecipitation kit (EpibiotekTM, Cat#R1819). 40 μL of protein G beads were washed twice with IP buffer and added into the lysate together with the antibody, followed by incubation overnight at 4 °C. After incubation, the supernatant was transferred into a fresh 1.5 mL tube. The beads were recovered by magnet and resuspended within 1× wash buffer, rotated at 4 °C for 10 min. The supernatant was removed and the washing step was repeated three times. Co-precipitated RNA was extracted by TRIzol™ Reagent (Invitrogen™, Cat# 15596018) and Phenol-chloroform method. Co-precipitated RNA and input RNA were subjected to library construction by using EpiTM mini longRNA-seq kit (Epibiotek, Cat# E1802) according to the manufacturer’s protocols. Briefly, reverse transcription was performed using random primers, and the ribosome cDNA (cDNA fragments originating from rRNA molecules) was removed after cDNA synthesis using probes specific to mammalian rRNA. The directionality of the template-switching reaction not only preserves the 5’ end sequence information of RNA but also the strand orientation of the original RNA. Libraries for immunoprecipitated RNA were PCR amplified for 18 cycles. Library quality was determined using Qseq100 Bio-Fragment Analyzer (Bioptic Inc.). The strand-specific libraries were sequenced on the Illumina Novaseq 6000 system with paired-end 2 × 150 bp read length.
RIP-qPCR was analyzed as previously described99. At least 3.0 × 107 macrophages were collected, was resuspended in 800 μL of lysis buffer (150 mM KCl, 20 mM Tris–HCl pH 7.5, 2 mM EDTA, 2 mM EDTA, 0.5% NP-40 0.5% Triton-100, 0.5 mM DTT, 1:100 protease inhibitor cocktail and 1:100 RNase inhibitor). Lysates were placed on ice for 15 min to remove cell debris. The lysates of 50 μL were kept for input. Remained lysates were mixed with anti-rabbit IgG magnetic beads (Thermo) and incubated with either 2 uL of anti-Mettl14 antibody or rabbit IgG overnight at 4 °C. Beads were washed with lysis buffer five times for 10 min. RNA was isolated from the beads by using trizol. RNAs were added with 1 μL glycogen and 1/10 volume, 3 M NaAc, and 1 volume isopropanol. The extracted RNAs were washed with 1 mL 75% ethanol once and added H2O to the pellet to elute the RNA, then reverse transcribed to cDNA for qPCR.
In vitro LLPS assay
GFP-METTL14, GFP-METTL14T72A, METTL14-6 × His, METTL14T72A-6 × His and METTL14T72D-6 × His were purified from E.coli BL21. As for the LLPS of phosphorylated METTL14, METTL14-6 × His and METTL14T72A-6 × His were first phosphorylated via “in vitro kinase assay” as shown above. Then purified proteins or p-METTL14 were incubated in LLPS buffer (50 mM Tris, PH = 7.5, 10% (w/v) PEG 3550 (Sigma), 2 mM DTT and 0.1% BSA (w/v)) for 30 min at room temperature with or without 10% (w/v) 1,6-Hexanediol (Sigma). And then 5 μL of the sample was pipetted onto a coverslip and image using a Leica SP8 microscope with differential interference contrast (DIC).
FRAP assay
The PFRP assay was performed using the FRAP module of the Leica SP8 confocal microscopy system. Briefly, GFP-METTL14 was bleached using a 488-nm laser beam. Bleaching was focused on a circular region of interest (ROI). After photobleaching, time-lapse images were captured. For each indicated time point (t), the fluorescence intensity within the bleached droplet was normalized to the fluorescence intensity of a nearby unbleached droplet. The normalized fluorescence intensity of pre-bleaching was set to 100%, and the normalized fluorescence intensity at each time point (It) was used to calculate the fluorescence recovery (FR) according to the following formula: FR(t) = It/I pre-bleaching. Image J was used for quantification and GraphPad Prism to plot and analyze the FRAP experiments.
Flow cytometry
For analysis of macrophage markers of peritoneal macrophage from mMettl14f/f and mMettl14−/− mice, cells were blocked by anti-CD16/32 (Biolegend, S17011E) for 15 min at 4 °C firstly. Then anti-F4/80 (Biolegend, BM8), anti-CD86 (Biolegend, GL-1), and anti-MHC II (Invitrogen, M5/114.15.2) were added to the preincubated cell and incubated at 4 °C for 30 min. Next, all the cells were fixed and permeabilized by the BD Fixation/Permeabilization kit (BD, 554714) following the instructions. After incubation with anti-CD206 (Biolegend, C068C2), the peritoneal macrophages were washed 3 times with PBS and analyzed by CytoFLEX S (Beckman). The data were collected and analyzed by FlowJo™ (BD).
In vitro infection of macrophages
For the in vitro infection of macrophages, non-adherent cells were removed by extensive washing with RPMI 1640. Bacteria were diluted and then incubated with cells for 2 h at 37 °C in 5% CO2. After washing twice, the infected cells were incubated in a fresh culture medium containing 50 μg/mL amikacin for 2 h. The infected cells were washed twice with PBS and lysed at 24 h with 0.1% (v/v) Triton X-100 in PBS. The number of viable intracellular bacteria (CFU) was determined by serial dilutions and plating out.
Mice and infection
C57BL/6J-Mettl14flox/flox mice were provided by Dr. Minghan Tong100 and were bred in SPF conditions at the Laboratory Animal Center of Tongji University. C57BL/6J-p38αflox/flox mice provided by Dr. Jiahuai Han101. C57BL/6J-Nox2−/− mice (Cat.# S-KO-01699) and C57BL/6J-Lyz2em1Cya (LysM-Cre) (Cat.# S-KO-03032) mice were purchased from Cyagen. To obtain conditional Mettl14-KO mice in myeloid cells (for short as mMettl14−/− in this study), 4-week-old mMettl14flox/flox female mice were mated with male C57BL/6J-Lyz2em1Cya mice. Similarly, 4-week-old p38αflox/flox female mice were mated with male C57BL/6J-Lyz2em1Cya mice to obtain conditional P38-KO mice in myeloid cells. Moreover, all animal experiments were reviewed and approved by the Animal Experiment Administration Committee of Tongji University School of Medicine.
The procedure for the generation of Mettl14 T72A knockin mice is similar to previous report102. The T7 promoter sequence was fused with the Cas9 coding region which was cloned from pX260 plasmid (Addgene #42229). Similarly, the T7 promoter sequence and the targeting sequence of STING were fused to the guide RNA scaffold which was cloned from the pX330 plasmid (Addgene # 42230). In vitro transcription of Cas9 mRNA and sgRNAs targeting Mettl14 was performed with mMESSAGE mMACHINE T7 ULTRA kit (ThermoFisher Scientific, AM1345, Waltham, MA) and MEGAshortscript T7 kit (ThermoFisher Scientific, AM1354, Waltham, MA) according to the manufacturer’s instructions, respectively. Both Cas9 mRNA and sgRNAs were then purified using MEGAclear Transcription Clean-Up Kit (Thermo Fisher Scientific, AM1908, Waltham, MA) and stored at −80 °C. One-cell embryos were collected from superovulated WT C57BL/6J female mice that had been mated with WT C57BL/6J male mice. Cas9 mRNA (50 ng/μL), sgRNA targeting Mettl14 (50 ng/μL), and donor listed in Supplementary Table S1 (ssODN, 100 ng/μL) were mixed together and then injected into the one-cell embryos. The injected embryos were cultured in EmbryoMax KSOM Medium (Sigma-Aldrich, MR-106-D, USA) until the two-cell stage, followed by transferring into the oviducts of recipients at 0.5 dpc. Recipient female mice delivered pups at 19.5 dpc. The first generation of point-mutant mice was identified using the genotyping primers listed in Supplementary Table S1. The mice harboring the correct point mutation were crossed for the expansion of the mouse population.
Infection studies were carried out using a murine respiratory infection model. Six-week-old female mice were divided randomly into cages and infected with 100–200 CFU of different H37Rv strains using an aerosol method at the Biosafety Level-3 (BSL-3) Laboratory. At 28 days post-infection, the mice were killed, and bacterial counts in the lungs were determined by plating tenfold serial dilutions of each tissue homogenate on Middlebrook 7H10 agar plates. For histology, lung tissues from H37Rv-infected mice were fixed in 4% PFA and then stained with H&E or Ziehl–Neelsen stain (acid-fast). The stained slides were visualized by light microscopy.
Histopathology analysis
An overall histology score was assigned to the lungs of mice based on the extent of granulomatous inflammation as follows: 0 = no lesion,1 = minimal lesion (1%–10% area of tissue in the section involved), 2 = mild lesion (11%–30% area involved); 3 = moderate lesion (30%–50% area involved); 4 = marked lesion (50%–80% area involved), and 5 = severe lesion (>80% area involved).
Clinical samples, ethics approval, and consent to participate
All protocols were approved by the local ethics committee of Shanghai Pulmonary Hospital (permit number: K23-333Z), and signed informed consent was obtained from all subjects. All patients provided the written informed consent in accordance with the Declaration of Helsinki. Diagnosis of TB was based on clinical presentation and radiological findings (such as an X-ray or computed tomography (CT) scan) and was confirmed by a positive sputum culture. The test for the anti-human immunodeficiency virus (HIV) antibody was negative for all TB patients. The average age was 40 years, and 65% of the patients were male.
In TB-negative patients, the inclusion criteria were no history of previous TB or anti-mycobacterial treatments and no evidence of TB-related infiltration in chest X-rays. The average age was 35 years, and 60% of the controls were male.
Quantification of Mettl14 foci
To quantify Mettl14 foci in the nucleus, the number of nuclear foci with a diameter over 200 nm was counted. The number of Mettl14 foci in 30 cells was shown as one point in every bar graph. About 100 cells were quantified in all of our studies.
Statistical analysis
Statistical significance between groups was determined by two-tailed Student’s t-test, two-tailed analysis of variance followed by Bonferroni post hoc test or two-sided Mann–Whitney U-test. Differences were significant for P < 0.05. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Data availability
The main data supporting the findings of this study are available within the paper. Additional data are available from the corresponding authors upon reasonable request.
References
Chakaya, J. et al. Global Tuberculosis Report 2020—reflections on the global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 113, S7–S12 (2021).
Liu, C. H., Liu, H. & Ge, B. Innate immunity in tuberculosis: host defense vs. pathogen evasion. Cell. Mol. Immunol. 14, 963–975 (2017).
Buter, J. et al. Mycobacterium tuberculosis releases an antacid that remodels phagosomes. Nat. Chem. Biol. 15, 889–899 (2019).
Carranza, C. & Chavez-Galan, L. Several routes to the same destination: inhibition of phagosome-lysosome fusion by Mycobacterium tuberculosis. Am. J. Med. Sci. 357, 184–194 (2019).
Chai, Q., Lu, Z. & Liu, C. H. Host defense mechanisms against Mycobacterium tuberculosis. Cell. Mol. Life Sci. 77, 1859–1878 (2020).
Obregon-Henao, A. et al. Stable extracellular RNA fragments of Mycobacterium tuberculosis induce early apoptosis in human monocytes via a caspase-8 dependent mechanism. PLoS ONE 7, e29970 (2012).
Wang, L. et al. Host-mediated ubiquitination of a mycobacterial protein suppresses immunity. Nature 577, 682–688 (2020).
Ganguly, N., Siddiqui, I. & Sharma, P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis 88, 510–517 (2008).
Hsu, T. et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100, 12420–12425 (2003).
Fortune, S. M. et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl. Acad. Sci. USA 102, 10676–10681 (2005).
Hemmati, M. et al. Expression and purification of recombinant Mycobacterium tuberculosis (TB) antigens, ESAT-6, CFP-10 and ESAT- 6/CFP-10 and their diagnosis potential for detection of TB patients. Iran. Red Crescent Med. J. 13, 556–563 (2011).
Hill, P. C. et al. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J. Clin. Microbiol. 43, 2070–2074 (2005).
Abebe, F., Belay, M., Legesse, M., Mihret, A. & Franken, K. S. Association of ESAT-6/CFP-10-induced IFN-gamma, TNF-alpha and IL-10 with clinical tuberculosis: evidence from cohorts of pulmonary tuberculosis patients, household contacts and community controls in an endemic setting. Clin. Exp. Immunol. 189, 241–249 (2017).
Belogorodtsev, S. N., Nemkova, E. K., Stavitskaya, N. V. & Schwartz, Y. S. Pathogenic effects of M. tuberculosis-specific proteins ESAT-6 and CFP-10 in macrophage culture and in 3D-granulemogenesis model in vitro. Bull. Exp. Biol. Med. 171, 656–660 (2021).
Seghatoleslam, A., Hemmati, M., Ebadat, S., Movahedi, B. & Mostafavi-Pour, Z. Macrophage immune response suppression by recombinant Mycobacterium tuberculosis antigens, the ESAT-6, CFP-10, and ESAT-6/CFP-10 fusion proteins. Iran. J. Med. Sci. 41, 296–304 (2016).
Furuichi, Y., LaFiandra, A. & Shatkin, A. J. 5’-Terminal structure and mRNA stability. Nature 266, 235–239 (1977).
Roundtree, I. A., Evans, M. E., Pan, T. & He, C. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017).
Zhao, B. S., Roundtree, I. A. & He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18, 31–42 (2017).
Frye, M., Harada, B. T., Behm, M. & He, C. RNA modifications modulate gene expression during development. Science 361, 1346–1349 (2018).
Zhao, X. et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 24, 1403–1419 (2014).
Wang, X. et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
Meyer, K. D. et al. 5’ UTR m(6)A promotes cap-independent translation. Cell 163, 999–1010 (2015).
Wang, S. et al. Roles of RNA methylation by means of N(6)-methyladenosine (m(6)A) in human cancers. Cancer Lett. 408, 112–120 (2017).
Liu, J. et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586 (2020).
Robinson, M., Shah, P., Cui, Y. H. & He, Y. Y. The role of dynamic m(6) A RNA methylation in photobiology. Photochem. Photobiol. 95, 95–104 (2019).
Huang, H., Weng, H. & Chen, J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 37, 270–288 (2020).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e616 (2018).
Bergeron-Sandoval, L. P., Safaee, N. & Michnick, S. W. Mechanisms and consequences of macromolecular phase separation. Cell 165, 1067–1079 (2016).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Eid, S. A., Savelieff, M. G., Eid, A. A. & Feldman, E. L. Nox, Nox, are you there? The role of NADPH oxidases in the peripheral nervous system. Antioxid. Redox Signal. 37, 613–630 (2021).
Zou, J. et al. Deoxyelephantopin induces reactive oxygen species-mediated apoptosis and autophagy in human osteosarcoma cells. Cell Physiol. Biochem. 42, 1812–1821 (2017).
Begum, R. et al. NADPH oxidase family proteins: signaling dynamics to disease management. Cell. Mol. Immunol. 19, 660–686 (2022).
Taylor, J. P. & Tse, H. M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 48, 102159 (2021).
Hultqvist, M., Olsson, L. M., Gelderman, K. A. & Holmdahl, R. The protective role of ROS in autoimmune disease. Trends Immunol. 30, 201–208 (2009).
Lichinchi, G. et al. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20, 666–673 (2016).
Liu, J. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 (2014).
Yankova, E. et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601 (2021).
Xi, C. et al. Mettl14-driven senescence-associated secretory phenotype facilitates somatic cell reprogramming. Stem Cell Rep. 17, 1799–1809 (2022).
Clemens, D. L., Lee, B. Y. & Horwitz, M. A. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 177, 5644–5652 (1995).
Miller, J. L., Velmurugan, K., Cowan, M. J. & Briken, V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 6, e1000864 (2010).
MacMicking, J. D. Cell-autonomous effector mechanisms against Mycobacterium tuberculosis. Cold Spring Harb. Perspect. Med. 4, a018507 (2014).
Chen, T., Zhu, J., Wang, Y. H. & Hang, C. H. ROS-mediated mitochondrial dysfunction and ER stress contribute to compression-induced neuronal injury. Neuroscience 416, 268–280 (2019).
Stanley, S. A., Raghavan, S., Hwang, W. W. & Cox, J. S. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA 100, 13001–13006 (2003).
Guinn, K. M. et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51, 359–370 (2004).
Boggaram, V., Gottipati, K. R., Wang, X. & Samten, B. Early secreted antigenic target of 6 kDa (ESAT-6) protein of Mycobacterium tuberculosis induces interleukin-8 (IL-8) expression in lung epithelial cells via protein kinase signaling and reactive oxygen species. J. Biol. Chem. 288, 25500–25511 (2013).
Li, F. et al. Early secreted antigenic target 6-kDa from Mycobacterium tuberculosis enhanced the protective innate immunity of macrophages partially via HIF1alpha. Biochem. Biophys. Res. Commun. 522, 26–32 (2020).
Liu, W. et al. The involvement of NADPH oxidase-mediated ROS in cytokine secretion from macrophages induced by Mycobacterium tuberculosis ESAT-6. Inflammation 37, 880–892 (2014).
Fabri, M. et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 3, 104ra102 (2011).
Guo, S. et al. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med. Hypotheses 78, 389–392 (2012).
Feng, Y. et al. Continuous treatment with recombinant Mycobacterium tuberculosis CFP-10-ESAT-6 protein activated human monocyte while deactivated LPS-stimulated macrophage. Biochem. Biophys. Res. Commun. 365, 534–540 (2008).
Aktipis, S. & Panayotatos, N. A kinetic study on the mechanism of inhibition of RNA synthesis catalyzed by DNA-dependent RNA polymerase. Differences in inhibition by ethidium bromide, 3,8-diamino-6-ethylphenanthridinium bromide and actinomycin d. Biochim. Biophys. Acta 655, 278–290 (1981).
Tang, L. et al. Emerging perspectives of RNA N (6)-methyladenosine (m(6)A) modification on immunity and autoimmune diseases. Front. Immunol. 12, 630358 (2021).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Wang, K., Peng, J. & Yi, C. The m(6)A consensus motif provides a paradigm of epitranscriptomic studies. Biochemistry 60, 3410–3412 (2021).
Haque, N. & Hogg, J. R. Easier, better, faster, stronger: improved methods for RNA-protein interaction studies. Mol. Cell 62, 650–651 (2016).
Lan, Q. et al. The emerging roles of RNA m(6)A methylation and demethylation as critical regulators of tumorigenesis, drug sensitivity, and resistance. Cancer Res. 81, 3431–3440 (2021).
Huang, H. et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295 (2018).
Zhou, K. I. & Pan, T. An additional class of m(6)A readers. Nat. Cell Biol. 20, 230–232 (2018).
Zhao, Y., Shi, Y., Shen, H. & Xie, W. m(6)A-binding proteins: the emerging crucial performers in epigenetics. J. Hematol. Oncol. 13, 35 (2020).
Xiao, Y. et al. An elongation- and ligation-based qPCR amplification method for the radiolabeling-free detection of locus-specific N(6)-methyladenosine modification. Angew. Chem. Int. Ed. Engl. 57, 15995–16000 (2018).
Ries, R. J. et al. m(6)A enhances the phase separation potential of mRNA. Nature 571, 424–428 (2019).
Chai, Q., Wang, L., Liu, C. H. & Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 17, 901–913 (2020).
Zarubin, T. & Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 (2005).
Kirsch, K. et al. Co-regulation of the transcription controlling ATF2 phosphoswitch by JNK and p38. Nat. Commun. 11, 5769 (2020).
Burton, J. C., Antoniades, W., Okalova, J., Roos, M. M. & Grimsey, N. J. Atypical p38 signaling, activation, and implications for disease. Int. J. Mol. Sci. 22, 4183 (2021).
Ge, B. et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 295, 1291–1294 (2002).
Remy, G. et al. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cell Signal. 22, 660–667 (2010).
Zhang, Y. Y., Mei, Z. Q., Wu, J. W. & Wang, Z. X. Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38alpha in different phosphorylation states. J. Biol. Chem. 283, 26591–26601 (2008).
Kumar, S., Jiang, M. S., Adams, J. L. & Lee, J. C. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 263, 825–831 (1999).
Champion, P. A., Stanley, S. A., Champion, M. M., Brown, E. J. & Cox, J. S. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313, 1632–1636 (2006).
Du, J. et al. N(6)-adenosine methylation of Socs1 mRNA is required to sustain the negative feedback control of macrophage activation. Dev. Cell 55, 737–753.e737 (2020).
Fu, X. D. Non-coding RNA: a new frontier in regulatory biology. Natl. Sci. Rev 1, 190–204 (2014).
Zhao, B. S. et al. m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478 (2017).
Yue, Y., Liu, J. & He, C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 29, 1343–1355 (2015).
Fu, Y. & Zhuang, X. m(6)A-binding YTHDF proteins promote stress granule formation. Nat. Chem. Biol. 16, 955–963 (2020).
Wang, J. et al. Binding to m(6)A RNA promotes YTHDF2-mediated phase separation. Protein Cell 11, 304–307 (2020).
Han, D. et al. Dynamic assembly of the mRNA m6A methyltransferase complex is regulated by METTL3 phase separation. PLoS Biol. 20, e3001535 (2022).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
Xiang, S. et al. The LC domain of hnRNPA2 adopts similar conformations in hydrogel polymers, liquid-like droplets, and nuclei. Cell 163, 829–839 (2015).
Murray, D. T. et al. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171, 615–627.e616 (2017).
Hofweber, M. & Dormann, D. Friend or foe-post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 294, 7137–7150 (2019).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345.e28 (2020).
Scholler, E. et al. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. RNA 24, 499–512 (2018).
Wang, X. et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578 (2016).
Brodin, P. et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J. Biol. Chem. 280, 33953–33959 (2005).
Pagan, A. J. et al. mTOR-regulated mitochondrial metabolism limits mycobacterium-induced cytotoxicity. Cell 185, 3720–3738.e3713 (2022).
Liu, Q. et al. Association of CYBB polymorphisms with tuberculosis susceptibility in the Chinese Han population. Infect. Genet. Evol. 33, 169–175 (2015).
Bustamante, J. et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat. Immunol. 12, 213–221 (2011).
Khan, T. A. et al. A novel missense mutation in the NADPH binding domain of CYBB abolishes the NADPH oxidase activity in a male patient with increased susceptibility to infections. Microb. Pathog. 100, 163–169 (2016).
Zeng, Y. et al. Clinical and genetic characteristics of BCG disease in Chinese children: a retrospective study. J. Clin. Immunol. 43, 756–768 (2023).
Deng, M., Lv, X. D., Fang, Z. X., Xie, X. S. & Chen, W. Y. The blood transcriptional signature for active and latent tuberculosis. Infect. Drug Resist. 12, 321–328 (2019).
Zhang, T. P., Li, R., Wang, L. J., Huang, Q. & Li, H. M. Roles of the m6A methyltransferases METTL3, METTL14, and WTAP in pulmonary tuberculosis. Front. Immunol. 13, 992628 (2022).
Renshaw, P. S. et al. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277, 21598–21603 (2002).
Zhao, M. et al. CGAS is a micronucleophagy receptor for the clearance of micronuclei. Autophagy 17, 3976–3991 (2021).
Chen, C. Y., Ezzeddine, N. & Shyu, A. B. Messenger RNA half-life measurements in mammalian cells. Methods Enzymol. 448, 335–357 (2008).
Wu, Y. et al. N(6)-methyladenosine regulates maternal RNA maintenance in oocytes and timely RNA decay during mouse maternal-to-zygotic transition. Nat. Cell Biol. 24, 917–927 (2022).
Lin, Z. et al. Mettl3-/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Res. 27, 1216–1230 (2017).
Kang, Y. J. et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J. Immunol. 180, 5075–5082 (2008).
Wang, F. et al. Cytoplasmic PARP1 links the genome instability to the inhibition of antiviral immunity through PARylating cGAS. Mol. Cell 82, 2032–2049.e37 (2022).
Acknowledgements
We thank Prof. Minghan Tong (University of Chinese Academy of Sciences) for the Mettl14flox/flox mice. We thank Prof. Jiahuai Han (Xiamen University) for p38αflox/flox mice and MKK6 (E) plasmid. We thank members of B.Ge’s laboratory (Shanghai Key Laboratory of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China) for helpful discussions and technical assistance. This project was supported by grants from the National Key R&D Program of China (2023YFC2307300, 2022YFC2302900, and 2021YFA1300902); the National Natural Science Foundation of China (32188101, 32030038, 82122029, 82102400 and 82071776); the fellowship of China National Postdoctoral Program for Innovative Talents (BX2021215); the Most Important Clinical Discipline in Shanghai (2017ZZ02003); Shanghai Rising-Star Program (20QA1408400); “Chen Guang” project (19CG22) Shanghai Municipal Education Commission and Shanghai Education Development Foundation.
Author information
Authors and Affiliations
Contributions
B.X.G. and L.W. conceptualized this project; B.X.G. supervised the overall experiments. Y.W.G., Y.W., M.T.M., Y.J.D., H.Y. and J.W. developed the methodology. M.T.M., C.P. and F.W. performed mouse experiments and histopathology analysis. M.T.M. and H.Y.M. performed western blotting assay, qPCR, and confocal experiments. M.T.M. performed the in vitro kinase assay, primary macrophage infection, and CFU assay. M.T.M., F.T. and Y.W.G. performed the MeRIP-qPCR, RIP-seq, and analysis of the data. Y.J.D., H.Y.C. and Y.F.H. performed experiments related to cell culture, FRAP assay, in vitro LLPS assay, immunofluorescence, and flow cytometry-related analysis. M.T.M, J.M.L., Y.N.C., Y.F.H. and J.P.H. detected the ROS, mito-ROS and LDH of macrophages. X.C.H., R.J.Z. and L.R.L. cultured the H37Rv strain and generated the complementary strain. J.L. and X.N.Z. performed the SELECT assay. J.S.L. and B.R.C. constructed Mettl14T72A/T72A knock-in mice. R.Y.Y., P.W., and W.S. collected clinical samples. B.X.G. and L.W. wrote the manuscript, and all authors commented on the manuscript, data, and conclusion.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, M., Duan, Y., Peng, C. et al. Mycobacterium tuberculosis inhibits METTL14-mediated m6A methylation of Nox2 mRNA and suppresses anti-TB immunity. Cell Discov 10, 36 (2024). https://doi.org/10.1038/s41421-024-00653-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41421-024-00653-4