Abstract
A polymorphism causing deficiencies in Toll-interacting protein (TOLLIP), an inhibitory adaptor protein affecting endosomal trafficking, is associated with increased tuberculosis (TB) risk. It is, however, unclear how TOLLIP affects TB pathogenesis. Here we show that TB severity is increased in Tollip−/− mice, characterized by macrophage- and T cell-driven inflammation, foam cell formation and lipid accumulation. Tollip−/− alveolar macrophages (AM) specifically accumulated lipid and underwent necrosis. Transcriptional and protein analyses of Mycobacterium tuberculosis (Mtb)-infected, Tollip−/− AM revealed increased EIF2 signalling and downstream upregulation of the integrated stress response (ISR). These phenotypes were linked, as incubation of the Mtb lipid mycolic acid with Mtb-infected Tollip−/− AM activated the ISR and increased Mtb replication. Correspondingly, the ISR inhibitor, ISRIB, reduced Mtb numbers in AM and improved Mtb control, overcoming the inflammatory phenotype. In conclusion, targeting the ISR offers a promising target for host-directed anti-TB therapy towards improved Mtb control and reduced immunopathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Datasets generated in this study are available as Source data. Image data are available on Zenodo at https://doi.org/10.5281/zenodo.10475042. All sequencing data that support the findings of this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and are accessible through the GEO Series accession number GSE243818. Any other relevant data are available from the corresponding author on request. Source data are provided with this paper.
Code availability
All custom scripts have been made available at https://github.com/altman-lab/Shah_mouse_collab. Additional modified scripts can be accessed upon request.
References
Shah, J. A. et al. Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J. Immunol. 189, 1737–1746 (2012).
Shah, J. A. et al. A functional TOLLIP variant is associated with BCG-specific immune responses and tuberculosis. Am. J. Respir. Crit. Care Med. 196, 502–511 (2017).
Shah, J. A. et al. Genetic variation in toll-interacting protein is associated with leprosy susceptibility and cutaneous expression of interleukin 1 receptor antagonist. J. Infect. Dis. 213, 1189–1197 (2016).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Jongsma, M. L. et al. An ER-associated pathway defines endosomal architecture for controlled cargo transport. Cell 166, 152–166 (2016).
Chen, K., Yuan, R., Zhang, Y., Geng, S. & Li, L. Tollip deficiency alters atherosclerosis and steatosis by disrupting lipophagy. J. Am. Heart Assoc. 6, e004078 (2017).
Lu, K., Psakhye, I. & Jentsch, S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 158, 549–563 (2014).
Shah, J. A. et al. TOLLIP deficiency is associated with increased resistance to Legionella pneumophila pneumonia. Mucosal Immunol. 12, 1382–1390 (2019).
Burns, K. et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2, 346–351 (2000).
Ryan, T. A. et al. Tollip coordinates Parkin‐dependent trafficking of mitochondrial‐derived vesicles. EMBO J. 39, e102539 (2020).
Zellner, S., Schifferer, M. & Behrends, C. Systematically defining selective autophagy receptor-specific cargo using autophagosome content profiling. Mol. Cell 81, 1337–1354.e8 (2021).
Zhang, G. & Ghosh, S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277, 7059–7065 (2002).
Brissoni, B. et al. Intracellular trafficking of interieukin-1 receptor I requires Tollip. Curr. Biol. 16, 2265–2270 (2006).
Pokatayev, V. et al. Homeostatic regulation of STING protein at the resting state by stabilizer TOLLIP. Nat. Immunol. 21, 158–167 (2020).
Kowalski, E. J. A. & Li, L. Toll-interacting protein in resolving and non-resolving inflammation. Front. Immunol. 8, 511 (2017).
Noth, I. et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir. Med. 1, 309–317 (2013).
Li, M. et al. Tollip is a critical mediator of cerebral ischaemia–reperfusion injury. J. Pathol. 237, 249–262 (2015).
Zhi, H. et al. Tollip negatively regulates vascular smooth muscle cell-mediated neointima formation by suppressing akt‐dependent signaling. J. Am. Heart Assoc. 7, e006851 (2018).
Behar, S. M. et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 4, 279–287 (2011).
Roca, F. J. & Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153, 521–534 (2013).
Berg, R. D. et al. Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration. Cell 165, 139–152 (2016).
Tzelepis, F. et al. Mitochondrial cyclophilin D regulates T cell metabolic responses and disease tolerance to tuberculosis. Sci. Immunol. 3, eaar4135 (2018).
Feng, C. G. et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171, 4758–4764 (2003).
Bafica, A. et al. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202, 1715–1724 (2005).
Watson, R. O. et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17, 811–819 (2015).
Philips, J. A., Porto, M. C., Wang, H., Rubin, E. J. & Perrimon, N. ESCRT factors restrict mycobacterial growth. Proc. Natl Acad. Sci. USA 105, 3070–3075 (2008).
Roca, F. J., Whitworth, L. J., Redmond, S., Jones, A. A. & Ramakrishnan, L. TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial–lysosomal–endoplasmic reticulum circuit. Cell 178, 1344–1361 e1311 (2019).
Lovewell, R. R., Sassetti, C. M. & VanderVen, B. C. Chewing the fat: lipid metabolism and homeostasis during M. tuberculosis infection. Curr. Opin. Microbiol. 29, 30–36 (2016).
Peyron, P. et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 4, e1000204 (2008).
Lo, Y. L. S., Beckhouse, A. G., Boulus, S. L. & Wells, C. A. Diversification of TOLLIP isoforms in mouse and man. Mamm. Genome 20, 305–314 (2009).
Russell, D. G., Huang, L. & VanderVen, B. C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19, 291–304 (2019).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Cohen, S. B. et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24, 439–446.e4 (2018).
Moguche, A. O. et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med. 212, 715–728 (2015).
GTEx Consortium. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
Nwosu, Z. C., Ebert, M. P., Dooley, S. & Meyer, C. Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Mol. Cancer 15, 71 (2016).
Stutz, M. D. et al. Macrophage and neutrophil death programs differentially confer resistance to tuberculosis. Immunity 54, 1758–1771.e7 (2021).
Joshi, N., Walter, J. M. & Misharin, A. V. Alveolar macrophages. Cell. Immunol. 330, 86–90 (2018).
Lavin, Y., Mortha, A., Rahman, A. & Merad, M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 15, 731–744 (2015).
Krämer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50, D687–D692 (2022).
Hinnebusch, A. G. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. Cold Spring Harb. Monogr. Ser. 39, 185–244 (2000).
Rabouw, H. H. et al. Inhibition of the integrated stress response by viral proteins that block p-eIF2–eIF2B association. Nat. Microbiol. 5, 1361–1373 (2020).
Agarwal, P., Gordon, S. & Martinez, F. O. Foam cell macrophages in tuberculosis. Front. Immunol. 12, 775326 (2021).
Bhattacharya, B. et al. The integrated stress response mediates necrosis in murine Mycobacterium tuberculosis granulomas. J. Clin. Invest. 131, e130319 (2020).
Vermeulen, I. et al. Mycolates of Mycobacterium tuberculosis modulate the flow of cholesterol for bacillary proliferation in murine macrophages. J. Lipid Res. 58, 709–718 (2017).
Zhu, X. G. et al. CHP1 regulates compartmentalized glycerolipid synthesis by activating GPAT4. Mol. Cell 74, 45–58.e7 (2019).
Ouimet, M. et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 13, 655–667 (2011).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17, 1–382 (2021).
Grootjans, J., Kaser, A., Kaufman, R. J. & Blumberg, R. S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484 (2016).
Kim, M. J. et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med. 2, 258–274 (2010).
Berry, M. P. R. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Volmer, R. & Ron, D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Biol. 33, 67–73 (2015).
Fu, S. et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011).
Krzyzosiak, A. et al. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell 174, 1216–1228.e9 (2018).
Rabouw, H. H. et al. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc. Natl Acad. Sci. USA 116, 2097–2102 (2019).
Han, J. & Kaufman, R. J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 57, 1329–1338 (2016).
Qin, S., Yin, J. & Huang, K. Free fatty acids increase intracellular lipid accumulation and oxidative stress by modulating PPARα and SREBP-1c in L-02 Cells. Lipids 51, 797–805 (2016).
Fineran, P. et al. Pathogenic mycobacteria achieve cellular persistence by inhibiting the Niemann-Pick Type C disease cellular pathway. Wellcome Open Res. 1, 18 (2016).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12, 352–366 (2012).
Raines, L. N. et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 23, 431–445 (2022).
Cornil, V. & Ranvier, L. A Manual of Pathological Histology, Translated, With Notes And Additions by E.O. Shakespeare and J. Henry C. Simes 394–445 (H. Kimpton, 1880).
Im, J. G., Itoh, H., Lee, K. S. & Han, M. C. CT-pathology correlation of pulmonary tuberculosis. Crit. Rev. Diagn. Imaging 36, 227–285 (1995).
Hunter, R. L. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 97, 8–17 (2016).
Gerner, M. Y., Kastenmuller, W., Ifrim, I., Kabat, J. & Germain, R. N. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376 (2012).
McQuattie-Pimentel, A. C. et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Invest. 131, e140299 (2021).
Montague, T. G., Cruz, J. M., Gagnon, J. A., Church, G. M. & Valen, E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401–W407 (2014).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Durinck, S. et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440 (2005).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Acknowledgements
We thank the University of Washington Center for Lung Biology Histology and Imaging Core for helpful advice on pathology staining and analysis; the Seattle Children’s Research Institute Cell Sorting Core for assistance and technical support; L. Ramakrishnan for helpful conversations and M. Sabo for copy editing. Flow cytometry data were acquired through the University of Washington, Cell Analysis Facility Shared Resource Lab, with NIH award 1S10 OD024979-01A1. This work was supported by the NIH (R01 AI136912 to J.A.S.; R01 DK108921 to S.A.S.) and the Department of Veterans Affairs (I01 BX004444 to S.A.S.). G.L.P. was supported by the American Diabetes Association (19-PDF-063).
Author information
Authors and Affiliations
Contributions
S.V., K.B.U., S.A.S. and J.A.S. conceptualized the project. S.V., S.A.S., K.B.U., M.A. and J.A.S. developed the methodology. J.A.S. and S.A.S. procured resources. K.A.D.-M. and M.A. developed software. S.V., C.R.P, S.B.C, J.A.S. and A.S. performed validation. M.A. K.A.D.-M. and B.H.G. conducted formal analysis. S.V., S.A.H., C.R.P., S.B.C, G.L.P., A.C.L., A.P., J.A.S., B.H.G., D.A.R., M.K.M., A.S., Y.-H.C. and C.F.H. conducted investigations. S.V. and J.A.S. wrote the original draft. K.B.U., S.A.S., M.A., D.A.R., C.R.P. and J.A.S. reviewed and edited the paper. J.A.S., K.B.U., M.A., S.A.S., B.H.G. and C.F.H. supervised the project. S.V. and J.A.S. administered the project. J.A.S., K.B.U., S.A.S., M.A. and B.H.G. acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Maziar Divangahi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Tollip−/− macrophages are hyperinflammatory.
a, b) Peritoneal extract macrophages (PEM) were isolated from Tollip−/− and B6 mice, plated, stimulated with control (Uns), LPS (10 ng/ml), PAM3 (250 ng/ml), or Mtb whole cell lysate (1 µg/ml) overnight and a) TNF and b) IL-10 were measured from cellular supernatants by ELISA. c–e) PEM were infected with Mtb H37Rv strain (MOI 2.5) overnight ex vivo, then c) TNF, d) IL-1β, and e) IL-10 were measured from cellular supernatants by ELISA. f–h) Baseline immune cell number in Tollip−/− mice. Total number of f) splenocytes, g) CD3+, C4 + CD8+, F4/80+, and NK1.1+ splenocytes, and the h) proportion of alveolar macrophages (AM) in the lungs of healthy 8-week-old mice, measured by flow cytometry. N = 3 measurements per experiment; each experiment was performed twice. * p < 0.05, student’s two sided t-test.
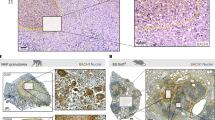
Extended Data Fig. 2 Related to Fig. 1. Characteristics of Mtb-infected Tollip−/− lungs.
a–g) Gating strategy used to identify Mtb-infected lung-resident myeloid cell subsets. a) Live/Dead Fixability dye was used to exclude dead cells, b) AM were identified by coexpression of SiglecF and CD11c. c) SiglecF- cells were gated; CD11b + Ly6G+ cells were classified as neutrophils, and CD11b + Ly6G- cells were considered macrophages. d) MHC-II+CD11c+ macrophages were subclassified as monocyte-derived macrophage cells (MDM) and MHC-II+CD11c- as interstitial macrophages (IM). e) CD11b-Ly6G- cells were gated and MHC-II+CD11c+ cells were classified as conventional DC (cDC) or f) CD103+ cDC) was measured. g) Representative flow cytometry images of the proportion of Mtb-infected myeloid subsets 28 and 56 days post infection (dpi). h–i) Proportion of AM, MDM, IM, PMN, and DC in the lungs h) 28 and i) 56 dpi. j) Representative images of Mtb-mCherry+ AM and MDM in Rag1−/− and Tollip−/−Rag1−/− mice.
Extended Data Fig. 3 Related to Fig. 2. Gating strategy used to identify lung-resident myeloid cells in mixed bone marrow chimeric mice.
a) Mixed bone marrow chimeric mice were infected with Mtb H37Rv expressing mCherry reporter plasmid (Mtb-mCherry) (50-100 CFU) via aerosol. At selected time points flow analysis was performed to identify populations infected with Mtb. Myeloid populations were identified as in Extended Data Fig. 2. a) Alveolar macrophages (AM), neutrophils (PMN), monocyte-derived macrophages (MDM), andinterstitial macrophages (IM) were subclassified as B6 or Tollip−/− based on CD45.1/CD45.2 or CD45.2 expression, respectively, and the proportion of mCherry-Mtb cells measured. Representative images from 28 days post infection are shown. b) Representative image of CD45.2+ Tollip−/− AM adoptively transferred into CD45.1+ mice 56 days after AM depletion.
Extended Data Fig. 4 Related to Fig. 3. Gating strategy used for sorting Mtb-infected AM.
Mixed bone marrow mice were infected with Mtb H37Rv (50-100 CFU) via aerosol and AM were sorted for RNA seq analysis 28 days post infection, from left to right. a) Lymphocyte gating, followed by b) Singlet identification. c) Live/Dead Fixability dye was used to exclude dead/dying cells, and d) SiglecF+ CD11c+ cells were classified as AM. e) Uninfected and Mtb-infected AM were identified by mCherry expression f) Uninfected AM and g) Mtb-infected AM genetic lineage was defined based on CD45.1/CD45.2 (F1 B6) or CD45.2 (Tollip−/−) expression, respectively.
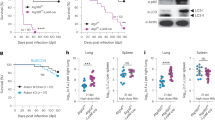
Extended Data Fig. 5 Related to Fig. 4. Tollip is dispensable for bulk autophagy in macrophages.
Bone marrow from B6 (white bars) or Tollip−/− (black bars) were differentiated ex vivo to macrophages using 40ng/mL M-CSF for 7-9 days. Following differentiation, BMDMs were treated for 6 hr with or without 250 nM Bafilomycin A (BafA). a) Representative western blot results showing protein levels of LC3I/II and p62. b) Quantification of protein levels (by densitometry) of LC3II using total LC3 (I + II) as a loading control. ***p < 0.001 2-way ANOVA for an effect of BafA. c) Quantification of p62 levels (by densitometry) using vinculin as a loading control. p = 0.056 2-way ANOVA effect of BafA. d–f) TNF concentrations in the supernatants of TOLLIP-deficient d) BMDM, e) alveolar macrophages (p = 0.02 between genotypes in Mtb and p = 0.03 in MA+Mtb groups; AM), and f) THP-1 cells 24 hours after Mtb infection (MOI 5) and mycolic acid (MA; 10 µg/ml) treatment by ELISA (p = 0.0002 between genotypes in Mtb and p < 0.0001 in MA+Mtb groups; AM)* p < 0.05, ** p < 0.01, *** p < 0.001, two-sided t-test. Experiment was performed in BMDM twice and all other cell types three times. g) Expression of EIF2AK1, EIF2AK2, EIF2AK4, and TOLLIP in human whole blood in heathy controls (control) or patients with latent tuberculosis infection (LTBI), active symptomatic pulmonary TB disease (Mtb). EIF2AK3 was not detected in this dataset. Data are shown as violin plots with lines indicating 25th, 50th, and 75th percentile, extending to minimum and maximum value. **** p < 0.0001, two-sided ANOVA. Obtained from GSE 1949152.
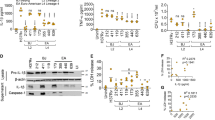
Extended Data Fig. 6 Related to Fig. 6. Evaluation of integrated stress responses in Tollip−/− mice and macrophages.
a–c) B6 and Tollip−/− peritoneal extract macrophages (PEM) were incubated with media or mycolic acid (MA; 10 µg/ml) for 72 hours, then infected with Mtb (MOI 1) overnight. mRNA transcripts of key regulatory genes of the cellular stress response were measured before and after Mtb infection. a) Ern1 (IRE1a; p = 0.001 for media, p = 0.049 for MA), b) Eif2ak3 (PERK; p = 0.002 for media, p = 0.007 for MA), and c) Atf6 (ATF6; p = 0.02 for media) were measured and displayed as their fold change from baseline after Mtb infection. FC = (Normalized mRNA expression after Mtb infection) / (Normalized mRNA expression after media control stimulation). N = 2/group and are representative of at least two independent experiments. d) Western blot of PEM incubated as above measuring pEIF2 and tubulin expression. e) Optical density at 600 nm (OD600) of Mtb H37Rv in 7H9 broth culture in the presence of raphin-1 (10 µM), ISRIB (250 nM), or vehicle control over time. N = 2 over two independent experiments; error bars – SEM. f–h) TNF concentrations in cellular supernatants from TOLLIP-deficient f) bone marrow-derived macrophages (BMDM), g) alveolar macrophages (AM; p = 0.04 for Mtb+MA, p = 0.003 for Mtb+ISRIB) and h) THP-1 cells (p = 0.002 for Mtb+MA, p = 0.005 for Mtb+ISRIB, p = 0.004 for Mtb+MA + ISRIB), after 24 hours of Mtb infection (MOI 5), mycolic acid (MA, 10 µg/ml), and ISRIB (250 nM), measured by ELISA. This experiment was performed twice, each with three technical replicates. * p < 0.05, ** p < 0.01, *** p < 0.001 two-sided t-test.
Extended Data Fig. 7 Images related to Fig. 6 histocytometry studies.
a) Representative confocal microscopy image of the lungs of a B6 mouse infected with Mtb for 56 days. Yellow – SiglecF; green – CD11b; red – pEIF2; blue – LipidTox; white — PPD. b) Histocytometry positional mapping of AM and MDM within the Mtb-infected lung. c) Proportion of pEIF2+ PPD + AM (p = 0.017 for overall ANOVA effect) and MDM (p = 0.016 for overall ANOVA effect) in B6 and Tollip−/− mice at baseline and after ISRIB treatment. p = 0.02 for overall ANOVA effect. * p < 0.05, two-sided ANOVA. N = 4 B6 control mice, N = 5 B6 ISRIB mice, N = 6 Tollip−/− control, and N = 6 Tollip−/− ISRIB mice. d) Representative images staining from the lungs of Mtb-infected B6 and Tollip−/− mice with and without ISRIB treatment. Red – pEIF2; green – CD68; blue – SiglecF; white – PPD. e) Spatial correlation analysis of cell types within 20-mm-radius neighborhoods in B6 and Tollip−/− mice. Red shades indicate positive correlation, and blue shades indicate negative correlation.
Extended Data Fig. 8 Overall experimental model.
a) Basal homeostasis. During chronic Mtb infection, lipid products are released in Mtb-infected AM. TOLLIP prevents lipid accumulation and controls inflammation, which maintains EIF2 signaling at a basal level. b) Tollip−/− AM. Tollip−/− mice develop excess TNF and IFNg responses from macrophages and T cells. Mtb-infected Tollip−/− AM undergo lipid accumulation, which increases EIF2 phosphorylation. Excess pEIF2 induces sensitivity to inflammation in AM. c) Chronic infection in Tollip−/− AM. During prolonged infection, increased and prolonged EIF2 phosphorylation from lipids and inflammation leads to cellular necrosis, decreasing the Mtb burden within individual AM and releasing extracellular Mtb. d) ISRIB treatment. ISRIB improves AM host defense, which improves Mtb control in both B6 and Tollip−/− mouse models, making it an effective therapeutic across genetic backgrounds.
Supplementary information
Supplementary Information
Extended Data figure legends 1–8, Source Data legends 1–7, Extended Data Source Data figure legends 1–5.
Supplementary Table 1
List of study resources.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Source data related to RNA-seq experiments, including differentially expressed genes, measures of effect size and statistical significance.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data, with uncropped western blots.
Source Data Extended Data Fig. 6
Statistical source data, with uncropped western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venkatasubramanian, S., Plumlee, C.R., Dill-McFarland, K.A. et al. TOLLIP inhibits lipid accumulation and the integrated stress response in alveolar macrophages to control Mycobacterium tuberculosis infection. Nat Microbiol 9, 949–963 (2024). https://doi.org/10.1038/s41564-024-01641-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01641-w