Abstract
When a cell dies of apoptosis, it is eliminated either by neighbouring cells or by attracted professional phagocytes. Although it was generally believed that neutrophils also have the ability to perform efferocytosis, their contribution to the clearance of apoptotic cells was considered less important compared with macrophages. Therefore, this ability of neutrophils remained unexplored for a long time. Over the past decade, it has been shown that during inflammation, neutrophils contribute significantly to the clearance of apoptotic neutrophils that accumulate in large numbers at the site of tissue damage. This “neutrophil cannibalism” is accompanied by inhibition of pro-inflammatory activities of these cells, such as respiratory burst and formation of neutrophil extracellular traps (NETs). Furthermore, efferocytosing neutrophils secrete anti-inflammatory mediators and mitogens including hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF2), vascular endothelial growth factors (VEGF), and transforming growth factor beta (TGFβ). Thus, efferocytosis by neutrophils is involved in resolution of inflammation. Recent research indicates that it plays also a role in cancer. Many different solid tumours contain aggregates of dead tumour cells that have undergone spontaneous apoptosis. Their extent correlates with poor clinical outcome in most cancer types. These clusters of apoptotic tumour cells are strongly infiltrated by tumour-associated neutrophils (TANs) that acquired an anti-inflammatory and pro-resolving polarization state. This review summarizes the potential consequences discussed in the current literature. Although the picture of the role of efferocytosis by neutrophils in inflammation and cancer is becoming clearer, many questions are still unexplored.
Similar content being viewed by others
FACTS
-
Apoptotic cells release “find-me” signals which attract predominantly neutrophils.
-
Neutrophils accumulate in areas of tissue damage and apoptotic tumour cells.
-
After engulfment of apoptotic cells, neutrophils block respiratory burst and NETosis.
-
Efferocytosing neutrophils secrete a variety of soluble mediators such as cytokines, chemokines, and mitogens which create a pro-resolving and tumorigenic microenvironment.
OPEN QUESTIONS
-
Several of the molecules known to be involved in the process of efferocytosis in macrophages are also expressed in neutrophils, but for many of them there is still a lack of evidence that they also fulfil this function there.
-
The exact mechanism by which neutrophils adopt a regenerative and tumourigenic phenotype after the uptake of apoptotic cells is still largely unexplored.
Neutrophils in inflammation
Neutrophils represent the first line of cellular innate immune response to infection and tissue damage. Recent evidence indicate that this short-lived myeloid cell population exhibits a great phenotypic and functional diversity [1]. It not only plays an important role in triggering inflammation in reaction to pathogens, but also contributes to its subsequent resolution after their clearance. Neutrophils accumulate quickly at the site of tissue damage through a multi-step process called “neutrophil swarming” [2]. Damage-associated molecular patterns (DAMPs) activate resident cells to release short-range chemoattractants for neutrophils. Pioneer neutrophils from around the damage site migrate to the tissue injury within minutes. The contact with pathogen-associated molecular patterns (PAMPs) stimulates them to deploy a plethora of antimicrobial weapons [3]. They form neutrophil extracellular traps (NETs) to entrap invading pathogens. They release a variety of antimicrobial and pro-inflammatory molecules from their granules and produce reactive oxygen species to kill bacteria. Finally, they clear pathogens by phagocytosis. Neutrophil-derived leukotriene B4 (LTB4) enhances the radius of recruitment of further neutrophils from distant tissue sites [2]. Notably, neutrophils also support the resolution of inflammation right from the start. They release anti-inflammatory, resolving and angiogenic mediators such as IL-10, transforming growth factor β (TGFβ), lipoxin 4A, resolvins, protectins, defensins, and vascular endothelial growth factor (VEGF) [4]. Neutrophils that have engulfed pathogens die through phagocytosis-induced apoptosis [5]. Cytokine receptors such as IL-1R on their surface, which are no longer functional, scavenge their pro-inflammatory ligands from the microenvironment [6]. Neighbourhood macrophages that phagocytose dying neutrophils adopt an anti-inflammatory, resolving and reparative M2-like phenotype [7,8,9]. Such resolving mechanisms begin to gain the upper hand as soon as the infection is pushed back. The accumulation of neutrophils at the site of tissue damage thus enables the restructuring of the extracellular matrix, the formation of dense aggregates that seal the wound tightly, and finally the initiation of tissue repair processes. It must be emphasized that signals of tissue damage are sufficient to trigger the attraction of neutrophils, which accordingly can also be observed in response to sterile injury without pathogens [3].
Efferocytosis by neutrophils
Especially during the early phase of neutrophil swarming, the number of resident macrophages is still very low and probably insufficient to clear all apoptotic neutrophils. Kristina Rydell-Törmänen showed in a mouse model of sterile lung inflammation that almost 50% of neutrophils at the side of injury have phagosomes that contained material from other neutrophils [10]. The authors termed this process “neutrophil cannibalism”. The efferocytotic capacity of neutrophils is similar to that of blood derived DCs, but clearly lower as compared to blood-derived macrophages [11]. It increases in response to pro-inflammatory cytokines such as TNF-α, interferon-gamma (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) and to ligands of TLR2 (Malp2, Pam3CSK4), TLR4 (LPS), TLR7/TLR8 (R848), and TLR9 (ODN 2006) [11, 12]. The efferocytotic ability of neutrophils is not exclusively limited to apoptotic conspecifics but also includes remnants of other cell types.
Detection of apoptotic cells
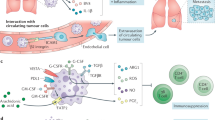
Apoptotic cells in general release various “find-me” signals that specifically attract neutrophils, including CCL3, CXCL1, CXCL5, CXCL8/IL8, tyrosyl tRNA synthetase (TyrRS) and endothelial monocyte activating polypeptide II (EMAPII) [13,14,15] (Fig. 1). This suggests that neutrophils may be intentionally recruited to help clear apoptotic cell debris. It has to be noted that apoptotic cells release also lactoferrin, which inhibits neutrophil migration [16]. However, this “keep-out” signal seems not to be sufficient to antagonize other chemoattractants in vivo. Garg and co-workers induced apoptotic cell death in a lung carcinoma cell line before injecting them intradermally into the mice ear pinna [17]. Cells exposed to the immunogenic apoptosis inducer mitoxantrone stimulated rapid recruitment of neutrophils, which in comparison to other leucocyte subsets constituted the predominant immune cell population accumulating at sites of apoptosis. Similarly, neutrophils accumulate at sites of apoptotic hepatocytes of patients with hepatocellular carcinoma [18].
In response to sterile injury or local infection, neutrophils migrate to the site of tissue damage in a multistep process termed “neutrophils swarming”. Neutrophils undergo apoptosis after phagocytosis of invading pathogens and contribute to local apoptotic cells. Apoptotic cells release “keep-out” signals as well as “find-me” signals, many of which are strong chemoattractants for neutrophils. Neutrophils detect “find-me” signals on the surface of apoptotic cells and engulf the cell remains. This leads to a blockade of signalling pathways responsible for respiratory burst and NETosis. Furthermore, efferocytosing neutrophils expose specific cell surface activation markers and secrete a variety of soluble mediators.
The predominant “eat me” signal on the surface of apoptotic cells is phosphatidylserine (PS). There is an extensive literature on the different mechanisms that macrophages use to detect PS-positive cell debris (reviewed in [8, 19]). They include directly binding receptors such as adhesion G protein-coupled receptor B1 (ADGRB1), stabilin-2 or T-cell membrane protein 4 (TIM-4). Furthermore, PS is also detected indirectly via soluble “bridging factors” like growth-arrest-specific gene-6 (GAS6) or Milk fat globule-EGF factor 8 protein (MFG-E8), which bind to PS and are then themselves detected by specific receptors on the macrophage. The MFG-E8 receptor αVβ3 integrin is also highly expressed in neutrophils [20]. However, neutrophils do not express any direct PS receptor (ADGRB1, stabilin-2, or TIM-4) or any receptor of GAS6. Besides PS, there are also other ‘eat-me’ signals exposed on apoptotic cells, including calreticulin, annexin A1, thrombospondin 1 binding sites, and complement proteins C1q or C3b binding sites [21]. They are recognized by CD91, formyl peptide receptor 2, CD47, CD93, and CD35, respectively. All of them are highly expressed in neutrophils [22,23,24,25,26]. However, their functional role in efferocytosis by these cells is still unexplored.
Clearance of apoptotic cells
The subsequent events in efferocytosis comprise the engulfment of apoptotic cellular corpses, followed by the formation and maturation of the phagosome, culminating with the degradation of the cargo within the phagolysosomal compartment. The molecular mechanisms of this tightly regulated multi-step process have been investigated in detail in macrophages (reviewed in [27]). After activation of “eat-me” receptors, the submembranous actin cortex undergoes specific rearrangements which activates the Rho family of small GTPase RAC1 and promotes the formation of a phagosome [27, 28]. The processing of engulfed cellular cargo requires a non-canonical LC3-asscociated phagocytosis (LAP) [29]. LAP represents a specialized mechanism that utilizes components of the autophagic machinery to enhance the degradation of phagocytosed material in an immunologically silent manner [30]. LAP is triggered by the recruitment of LC3 (microtubule-associated protein 1A/1B-light chain 3) proteins to the single-membrane phagosomes (or LAPosome). For that, PI3KC3 complex needs to be assembled, which consists of Rubicon, vps34, beclin-1, and vps15. This complex converts the LAPosome-bound phosphatidylinositol into the signalling lipid phosphatidylinositol 3-phosphate (PI3P) [27, 28]. The PI3P-coated LAPosome stabilizes the NOX2 complex which is responsible for ROS generation, leading to LC3 ligation machinery activation and LC3-II recruitment to the LAPosome [31]. This last step facilitates the LAPosme-lysosome fusion, resulting in a rapid degradation of the cargo. Recently, Prajsnar et al. identified the LAP machinery in neutrophils, but unfortunately its activation upon efferocytosis of apoptotic cells has not been investigated [32].
Cunha et al. noted that engulfment of apoptotic corpses per se does not result in immunosuppression, but it is rather the subsequent accumulation of digested products which induces immune tolerance [33]. The phagocytes overload with lipids and cholesterol stimulates the activation of nuclear steroid receptors from the liver X receptors (LXRs) and peroxisome proliferator-activated receptors (PPARs) families [34, 35]. Apart from mediating lipid homoeostasis, LXRs and PPARs induce the clearance of apoptotic cells via expression of phagocytic receptors and opsonins, resembling a positive feedback loop. The anti-inflammatory effects attributed to efferocytosis are also mediated by these pathways, by promoting upregulation of the anti-inflammatory cytokines TGFβ and IL-10 whereas the pro-inflammatory cytokines TNFα, IL-1β, and IL-6 are downregulated [19]. A similar response has been observed in neutrophils that had phagocytosed apoptotic neutrophils [11, 36]. They showed an elevated expression of anti-inflammatory TGFβ and of neutrophil chemoattractants CXCL1 and CXCL8/IL8, and a lower secretion of pro-inflammatory cytokines TNFα and CXCL10/IP-10. Furthermore, they downregulate respiratory burst due to a reduced phosphorylation of p38 MAPK and PKCδ, the kinases involved in NADPH oxidase activation [37]. Incubation with anti-TGFβ1 antibodies restores respiratory burst [36]. The inhibitory effect of neutrophil cannibalism on respiratory burst is exploited by invading bacteria to their own advantage. For instance, Leishmania major-infected neutrophils acquire enhanced capacity to engulf apoptotic cells. The uptake of apoptotic cells inhibits respiratory burst, protecting thereby the bacteria [37]. Manfredi et al. found that MPO and elastase are translocated into phagolysosomes during the process of efferocytosis to facilitate cargo degradation, making these enzymes unavailable for participating in chromatin decondensation – a prerequisite for NET formation [38]. Thus, neutrophilic efferocytosis impedes NETosis and primes these cells towards a non-inflammatory and resolving phenotype. We could demonstrate recently that neutrophils engulf apoptotic cell-derived extracellular vesicles (aEV) from hepatocytes and several cancer cell lines [39]. This is associated with an increase of cell surface activation markers CD11b, CD16, CD45, CD66b, CD62L, and secretion of various mitogens, including hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF2), VEGF, and transforming growth factor alpha (TGFα). Neutrophils express HGF mRNA and store the active protein in secretory vesicles and gelatinase granules [40]. The release of HGF and other mitogens in response to aEV results in an elevated metabolic activity and proliferation of co-cultured hepatocytes [39]. This indicates that efferocytosing neutrophils induce tissue regeneration in response to an uptake of apoptotic cells. Strong corroboration for this hypothesis comes from an observation in patients undergoing partial hepatectomy, a surgical procedure that results in massive local apoptosis at the resection margins of the remaining liver lobes [39]. Free HGF as well as neutrophil-bound HGF in the circulation of these patients correlate with the degree of apoptosis. Notably, higher levels of HGF are associated with improved liver regeneration.
Neutrophils in cancer
Fridlender et al. identified two distinct populations of tumour-associated neutrophils (TANs): anti-tumourigenic N1 and pro-tumourigenic N2 TANs [41]. The latter type prevails in many human cancers [42]. It releases a variety of cytokines, chemokines, and growth factors that promote tumour cell survival and proliferation, such as prostaglandin E2 (PGE2), CCL17, interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), VEGF, and epidermal growth factor (EGF) (Fig. 2) [43]. N2 TANs also secrete collagenase (MMP8) and gelatinase B (MMP9), which facilitate the invasion of tumour cells by remodelling the extracellular matrix [44]. In addition, their arginase-1 (ARG1) degrades extracellular arginine, which dampens the proliferation of T cells [45]. Thus, N2 TANs resemble in many respects to neutrophils after uptake of apoptotic cells.
Spontaneous or therapy-induced tumour apoptosis leads to attraction of tumour-associated neutrophils (TANs). They are exposed to tumour-derived factors (dotted line) and engulf apoptotic cell remains. Both polarizes efferocytosing neutrophils towards an anti-inflammatory and pro-resolving N2-like phenotype. These TANs secrete numerous soluble mediators, which modulate tumour cells (Tu), tumour-associated macrophages (TAMs), tumour infiltrating lymphocytes (TILs), endothelial cells (ECs) and the extracellular matrix (ECM) in a pro-tumourigenic way. In addition, cancer may intravasate into adjacent vessels resulting in circulating tumour cells (CTCs). The blood stream is a harsh environment and many of CTCs have a short half-life. CTCs may form clusters with high abundant blood cells such as platelets or neutrophils (polymorphonuclear leucocytes or PMNs). They form NETs protecting CTCs and supporting extravasation and metastasis. Apoptotic CTCs within clusters are expected to bind neutrophils for efferocytosis, which would further support CTC survival and proliferation. However, experimental proof for this role of efferocytosis by neutrophils is still pending.
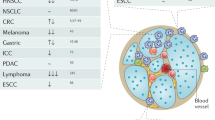
Spontaneous apoptosis of single tumour cells can be observed in many treatment-naive patients. It was shown already more than 25 years ago in prostate cancer that an elevated frequency of tumour cell apoptosis correlates with a higher 5-years progression rate [46]. Similarly, colorectal cancer patients with a higher number of apoptotic cancer cells have a worse overall survival [47]. Table 1 summarizes numerous studies investigating the relationship between cancer apoptosis rate and clinical outcome in 18 different cancer types. A positive association between apoptosis and poor prognosis was found in most cancers types. Only thyroid carcinoma, neuroblastoma, and glioblastoma showed an inverse relationship. Thus, tumour cell apoptosis promotes the progression of remaining viable tumour cells. Apoptotic cells release the growth factor FGF-β, PGE2, and VEGF, which have a direct promoting effect on the proliferation of adjacent tumour cells (recently reviewed in [48]). However, there is strong evidence that the pro-tumourigenic effect of apoptosis is mainly mediated by the phagocyte response during apoptotic cell clearance [49]. Most studies focussed on macrophages, whereas the contribution of efferocytosing neutrophils to tumour growth is much less investigated. Dead tumour cells are not equally distributed throughout the tumour tissue. Many solid cancers show dense cribriform nests or pseudoluminal structures with central aggregates of disintegrated dead tumour cells [50]. We found recently in colorectal cancer patients that such massive dead cell accumulations stain positive for caspase-cleaved cytokeratin 18 and CXCL8/IL-8, indicating that they derive from apoptotic tumour cells, which release a neutrophil chemoattractant (Fig. 2) [15]. Accordingly, the great majority of aggregates is highly infiltrated with neutrophils and anti-inflammatory polarized TAMs. Blocking the apoptotic cell-derived CXCL8/IL-8 prevents neutrophil-induced anti-inflammatory macrophage polarization. These data fit to the above-proposed concept that neutrophils play a major role in efferocytosis in cases of massive accumulations of apoptotic dead cell remnants.
Interestingly, also activation of LAP promotes tumour immune tolerance. LAP-sufficient tumour animal models revealed accumulation of M2 macrophages which support the pro-tumorigenic effects of tumour-associated macrophages (TAMs) [33]. Consequently, T cell differentiation is skewed towards regulatory T cells that support inflammation resolution [19, 33]. Indeed, LAP-deficient TAMs trigger STING-mediated type I interferon responses inducing a pro-inflammatory gene expression and increasing CD8+ T cell function. Remarkably, the overexpression of Rubicon in cancer patients, which is required for LAP but not autophagy, have been suggested as a potential poor prognostic marker [51]. In line with that, evidence suggest that specifically targeting LAP within the tumour microenvironment through pharmaceutical means promotes an anti-tumour response in a T cell-dependent manner [33]. Hence, development of therapies targeting efferocytosis-related pathways, in macrophages as well as neutrophils, could present a promising approach for cancer treatment.
Neutrophils support tumour growth and spreading not only in the tissue but also in the blood stream. They form heterologous clusters with circulating tumour cells (CTCs) which prolongs their half-life (Fig. 2) [52]. CTC-neutrophils clusters support cell cycle progression, proliferation and survival of tumour cells resulting in extended metastatic potential [53]. Patients with CTC–neutrophil clusters have poorer outcomes compared to those with homotypic CTC clusters [54]. CTC-neutrophil clusters may also include NETs, which promote adhesion and extravasation of CTCs at the site of metastasis [55, 56]. However, sequencing analysis of CTC-associated neutrophils revealed a N2-like gene expression profile, indicating that not all neutrophils in the clusters form NETs [41]. N2 neutrophils offer CTCs protection from immune surveillance by inhibition of CD8+ T cells NK cells [57]. The blood-stream is a harsh environment for CTCs and single tumour cells may die quickly. They may be efferocytosed by adjacent neutrophils in the cluster, leading to mitogenic support of remaining tumour cell in the cluster. In summary, anti-inflammatory neutrophils support tumour growth and spreading in a variety of ways. Although there is growing evidence that efferocytosis contributes to tumorigenic TAN polarization, further research is needed to confirm this concept.
Conclusion and outlook
There is ample evidence that efferocytosis by neutrophils plays an important role in the response to dead cell accumulation. During inflammation, they contribute to the clearance of aggregates of apoptotic neutrophils. In cancer, they participate in the removal of dead tumour cell aggregates. The neutrophil response after engulfing apoptotic cells contributes to the resolution of inflammation and tissue regeneration. However, in the case of cancer, this can be harmful. A meta-analysis of expression signatures from more than 18,000 human tumours found that neutrophils are the tumour-associated cell type linked with the worst prognosis [58]. Neutrophilic efferocytosis might contribute to this situation. As professional phagocytes, neutrophils have the full machinery for engulfment and express many receptors for the detection and binding of dead cells. However, it is also still unclear whether neutrophils distinguish between the different types of cell death from which their target cells have died. Although an increasingly clear picture is emerging on efferocytosis by neutrophils, there are still many unanswered questions awaiting exploration.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40:565–83.
Kienle K, Lämmermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 2016;273:76–93.
Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, et al. Neutrophils: new insights and open questions. Sci Immunol 2018;3:eaat4579.
Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129:2629–39.
Kirschnek S, Ying S, Fischer SF, Häcker H, Villunger A, Hochrein H, et al. Phagocytosis-induced apoptosis in macrophages is mediated by up-regulation and activation of the Bcl-2 homology domain 3-only protein bim. J Immunol. 2005. https://doi.org/10.4049/jimmunol.174.2.671.
Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–45.
Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–74.
Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80.
Brostjan C, Oehler R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020;6:26.
Roth S, Agthe M, Eickhoff S, Möller S, Karsten CM, Borregaard N, et al. Secondary necrotic neutrophils release interleukin-16C and macrophage migration inhibitory factor from stores in the cytosol. Cell Death Discov. 2015;1:15056.
Esmann L, Idel C, Sarkar A, Hellberg L, Behnen M, Möller S, et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol. 2010;184:391–400.
Hellberg L, Fuchs S, Gericke C, Sarkar A, Behnen M, Solbach W, et al. Proinflammatory stimuli enhance phagocytosis of apoptotic cells by neutrophil granulocytes. ScientificWorldJournal. 2011;11:2230–6.
Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, et al. Fas/CD95-induced chemokines can serve as ‘find-me’ signals for apoptotic cells. Mol Cell. 2013;49:1034–48.
Medina CB, Ravichandran KS. Do not let death do us part: ‘find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016;23:979–89.
Schimek V, Strasser K, Beer A, Göber S, Walterskirchen N, Brostjan C, et al. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. 2022;13:113.
Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32.
Garg AD, Vandenberk L, Fang S, Fasche T, Van Eygen S, Maes J, et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017;24:832–43.
Xu LC, Ma L, Zhao J, Wang X, Fan X, Li W, et al. An unexpected role of neutrophils in clearing apoptotic hepatocytes in vivo. Elife. 2023;12:RP86591.
Doran AC, Yurdagul A, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol 2020;20:254–67.
Kim H-Y, Skokos EA, Myer DJ, Agaba P, Gonzalez AL. αVβ3 integrin regulation of respiratory burst in fibrinogen adherent human neutrophils. Cell Mol Bioeng. 2014;7:231–42.
Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. 2022;21:601–20.
Liberale L, Bertolotto M, Minetti S, Contini P, Verzola D, Ameri P, et al. Recombinant tissue plasminogen activator (r-tPA) induces in-vitro human neutrophil migration via low density lipoprotein receptor-related protein 1 (LRP-1). Int J Mol Sci; 2020;21. https://doi.org/10.3390/ijms21197014.
Spurr L, Nadkarni S, Pederzoli-Ribeil M, Goulding NJ, Perretti M, D’Acquisto F. Comparative analysis of Annexin A1-formyl peptide receptor 2/ALX expression in human leukocyte subsets. Int Immunopharmacol. 2011;11:55–66.
Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5.
Tossetta G, Piani F, Borghi C, Marzioni D. Role of CD93 in health and disease. Cells. 2023;12. https://doi.org/10.3390/cells12131778.
Vandendriessche S, Cambier S, Proost P, Marques PE. Complement receptors and their role in leukocyte recruitment and phagocytosis. Front cell Dev Biol. 2021;9:624025.
Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21:398–414.
Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016;23:1–12.
Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7.
Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci USA. 2011;108:17396–401.
Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906.
Prajsnar TK, Serba JJ, Dekker BM, Gibson JF, Masud S, Fleming A, et al. The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils. Autophagy. 2021;17:888–902.
Cunha LD, Yang M, Carter R, Guy C, Harris L, Crawford JC, et al. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell. 2018;175:429–441.e16.
A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58.
Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–72.
Steiger S, Harper JL. Neutrophil cannibalism triggers transforming growth factor β1 production and self regulation of neutrophil inflammatory function in monosodium urate monohydrate crystal-induced inflammation in mice. Arthritis Rheum. 2013;65:815–23.
Salei N, Hellberg L, Köhl J, Laskay T. Enhanced survival of Leishmania major in neutrophil granulocytes in the presence of apoptotic cells. PLoS One. 2017;12:e0171850.
Manfredi AA, Ramirez GA, Rovere-Querini P, Maugeri N. The neutrophil’s choice: phagocytose vs make neutrophil extracellular traps. Front Immunol. 2018;9:288.
Brandel V, Schimek V, Göber S, Hammond T, Brunnthaler L, Schrottmaier WC, et al. Hepatectomy-induced apoptotic extracellular vesicles stimulate neutrophils to secrete regenerative growth factors. J Hepatol. 2022;77:1619–30.
Grenier A, Chollet-Martin S, Crestani B, Delarche C, El Benna J, Boutten A, et al. Presence of a mobilizable intracellular pool of hepatocyte growth factor in human polymorphonuclear neutrophils. Blood. 2002;99:2997–3004.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell. 2009;16:183–94.
Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601–20.
Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503.
Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–7.
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004;64:5839–49.
Aihara M, Scardino PT, Truong LD, Wheeler TM, Goad JR, Yang G, et al. The frequency of apoptosis correlates with the prognosis of Gleason Grade 3 adenocarcinoma of the prostate. Cancer. 1995;75:522–9.
Rupa JD, De Bruïne AP, Gerbers AJ, Leers MPG, Nap M, Kessels AGH, et al. Simultaneous detection of apoptosis and proliferation in colorectal carcinoma by multiparameter flow cytometry allows separation of high and low-turnover tumors with distinct clinical outcome. Cancer. 2003;97:2404–11.
Eskandari E, Eaves CJ. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol. 2022;221. https://doi.org/10.1083/jcb.202201159.
Gregory CD, Ford CA, Voss JJLP. Microenvironmental effects of cell death in malignant disease. Adv Exp Med Biol. 2016;930:51–88.
Branca G, Ieni A, Barresi V, Tuccari G, Caruso RA. An updated review of cribriform carcinomas with emphasis on histopathological diagnosis and prognostic significance. Oncol Rev. 2017;11:317.
Asare PF, Roscioli E, Hurtado PR, Tran HB, Mah CY, Hodge S. LC3-associated phagocytosis (LAP): a potentially influential mediator of efferocytosis-related tumor progression and aggressiveness. Front Oncol. 2020;10:1298.
Zhang J, Qiao X, Shi H, Han X, Liu W, Tian X, et al. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumour Biol. 2016;37:5397–404.
Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–7.
Hattar K, Franz K, Ludwig M, Sibelius U, Wilhelm J, Lohmeyer J, et al. Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother. 2014;63:1297–306.
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–80.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–49.
Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–45.
Kiberu SW, Pringle JH, Sobolewski S, Murphy P, Lauder I. Correlation between apoptosis, proliferation and bcl-2 expression in malignant non-Hodgkin’s lymphoma. Clin Mol Pathol. 1996;49:M268–72.
Spina D, Leoncini L, Del Vecchio MT, Megha T, Minacci C, Poggi SA, et al. Low versus high cell turnover in diffusely growing non-Hodgkin’s lymphomas. J Pathol. 1995;177:335–41.
Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, Davaris PS. Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiology. 2001;69:266–73.
Lipponen P, Aaltomaa S, Kosma VM, Syrjänen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;30A:2068–73.
Vakkala M, Lähteenmäki K, Raunio H, Pääkkö P, Soini Y. Apoptosis during breast carcinoma progression. Clin Cancer Res. 1999;5:319–24.
Huang Q, Li F, Liu X, Li W, Shi W, Liu F-F, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–6.
Miyamoto M, Takano M, Iwaya K, Shinomiya N, Goto T, Kato M, et al. High-temperature-required protein A2 as a predictive marker for response to chemotherapy and prognosis in patients with high-grade serous ovarian cancers. Br J Cancer. 2017;116:e2.
Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, Kulesza-Bronczyk B, Kinalski M, Terlikowski SJ. Prognostic significance of pretreatment VEGF, survivin, and Smac/DIABLO serum levels in patients with serous ovarian carcinoma. Tumour Biol. 2015;36:4157–65.
Kleinberg L, Dong HP, Holth A, Risberg B, Trope’ CG, Nesland JM, et al. Cleaved caspase-3 and nuclear factor-kappaB p65 are prognostic factors in metastatic serous ovarian carcinoma. Hum Pathol. 2009;40:795–806.
Hu Q, Peng J, Liu W, He X, Cui L, Chen X, et al. Elevated cleaved caspase-3 is associated with shortened overall survival in several cancer types. Int J Clin Exp Pathol. 2014;7:5057–70.
McMenamin ME, O’Neill AJ, Gaffney EF. Extent of apoptosis in ovarian serous carcinoma: relation to mitotic and proliferative indices, p53 expression, and survival. Mol Pathol. 1997;50:242–6.
Baretton GB, Diebold J, Christoforis G, Vogt M, Müller C, Dopfer K, et al. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Aspects of carcinogenesis and prognostic significance. Cancer. 1996;77:255–64.
Langlois NE, Lamb J, Eremin O, Heys SD. Apoptosis in colorectal carcinoma occurring in patients aged 45 years and under: relationship to prognosis, mitosis, and immunohistochemical demonstration of p53, c-myc and bcl-2 protein products. J Pathol. 1997;182:392–7.
Törmänen U, Eerola AK, Rainio P, Vähäkangas K, Soini Y, Sormunen R, et al. Enhanced apoptosis predicts shortened survival in non-small cell lung carcinoma. Cancer Res. 1995;55:5595–602.
Tanaka F, Kawano Y, Li M, Takata T, Miyahara R, Yanagihara K, et al. Prognostic significance of apoptotic index in completely resected non-small-cell lung cancer. J Clin Oncol. 1999;17:2728–36.
Ikeguchi M, Cai J, Yamane N, Maeta M, Kaibara N. Clinical significance of spontaneous apoptosis in advanced gastric adenocarcinoma. Cancer. 1999;85:2329–35.
Aaltomaa S, Kärjä V, Lipponen P, Isotalo T, Kankkunen JP, Talja M, et al. Expression of Ki-67, cyclin D1 and apoptosis markers correlated with survival in prostate cancer patients treated by radical prostatectomy. Anticancer Res. 2006;26:4873–8.
Stapleton AM, Zbell P, Kattan MW, Yang G, Wheeler TM, Scardino PT, et al. Assessment of the biologic markers p53, Ki-67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998;82:168–75.
Ben-Izhak O, Laster Z, Araidy S, Nagler RM. TUNEL—an efficient prognosis predictor of salivary malignancies. Br J Cancer. 2007;96:1101–6.
Lipponen PK, Aaltomaa S. Apoptosis in bladder cancer as related to standard prognostic factors and prognosis. J Pathol. 1994;173:333–9.
Korkolopoulou P, Konstantinidou AE, Christodoulou P, Patsouris E, Thomas-Tsangli E, Kapralos P, et al. Apoptosis in bladder carcinomas detected with monoclonal antibody to single-stranded DNA: relation to cell cycle regulators and survival. Urology. 2000;56:516–20.
Meggiato T, Calabrese F, Valente M, Favaretto E, Baliello E, Del Favero G. Spontaneous apoptosis and proliferation in human pancreatic cancer. Pancreas. 2000;20:117–22.
Magistrelli P, Coppola R, Tonini G, Vincenzi B, Santini D, Borzomati D, et al. Apoptotic index or a combination of Bax/Bcl-2 expression correlate with survival after resection of pancreatic adenocarcinoma. J Cell Biochem. 2006;97:98–108.
Soini Y, Virkajärvi N, Lehto VP, Pääkkö P. Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer. 1996;73:1025–30.
Ito Y, Matsuura N, Sakon M, Takeda T, Umeshita K, Nagano H, et al. Both cell proliferation and apoptosis significantly predict shortened disease-free survival in hepatocellular carcinoma. Br J Cancer. 1999;81:747–51.
Gestblom C, Hoehner JC, Påhlman S. Proliferation and apoptosis in neuroblastoma: subdividing the mitosis-karyorrhexis index. Eur J Cancer. 1995;31A:458–63.
Beer TW, Carr NJ, Whittaker MA, Pullinger N. Mitotic and in situ end-labeling apoptotic indices as prognostic markers in malignant mesothelioma. Ann Diagn Pathol. 2000;4:143–8.
Kahlos K, Soini Y, Pääkkö P, Säily M, Linnainmaa K, Kinnula VL. Proliferation, apoptosis, and manganese superoxide dismutase in malignant mesothelioma. Int J cancer. 2000;88:37–43.
Naresh KN, Lakshminarayanan K, Pai SA, Borges AM. Apoptosis index is a predictor of metastatic phenotype in patients with early stage squamous carcinoma of the tongue: a hypothesis to support this paradoxical association. Cancer. 2001;91:578–84.
Hirvikoski P, Kumpulainen E, Virtaniemi J, Pirinen R, Salmi L, Halonen P, et al. Enhanced apoptosis correlates with poor survival in patients with laryngeal cancer but not with cell proliferation, bcl-2 or p53 expression. Eur J Cancer. 1999;35:231–7.
Kuriyama H, Lamborn KR, O’Fallon JR, Iturria N, Sebo T, Schaefer PL, et al. Prognostic significance of an apoptotic index and apoptosis/proliferation ratio for patients with high-grade astrocytomas. Neuro Oncol. 2002;4:179–86.
Acknowledgements
This study was supported by the Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
CR and RO performed writing, review and revision of the paper. Both authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramos, C., Oehler, R. Clearance of apoptotic cells by neutrophils in inflammation and cancer. Cell Death Discov. 10, 26 (2024). https://doi.org/10.1038/s41420-024-01809-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-01809-7