Abstract
Arsenic trioxide is a first-line treatment drug for acute promyelocytic leukemia, which is also effective for other kinds of leukemia. Its side effects, however, limit its clinical application, especially for patients with complex leukemia symptoms. Combination therapy can effectively alleviate these problems. This review summarizes the research progress on the combination of arsenic trioxide with anticancer drugs, vitamin and vitamin analogs, plant products, and other kinds of drugs in the treatment of leukemia. Additionally, the new progress in arsenic trioxide-induced cardiotoxicity was summarized. This review aims to provide new insights for the rational clinical application of arsenic trioxide.

Similar content being viewed by others
Facts
-
Arsenic trioxide is a first-line treatment drug for acute promyelocytic leukemia in clinic.
-
Arsenic trioxide can induce serious side effects, which limit its clinical application.
-
There were a lot of drug combination strategies for alleviating arsenic trioxide-induced side effects and improving the curative effects.
-
Combination therapy was becoming an achievable strategy for the treatment of leukemia with arsenic trioxide.
-
Plant products are more likely to be used in combination with arsenic trioxide.
-
Noncoding RNAs are involved in the mechanisms of the arsenic trioxide-induced cardiotoxicity.
Open questions
-
How should we do to find the efficient and pivotal combination therapy for alleviating arsenic trioxide-induced side effects, and improving its curative effects on leukemia?
-
What is the key therapeutic mechanism of combined medication?
-
What are the advantages of plant products combined with arsenic trioxide?
-
What regulatory role does noncoding RNA play in the therapeutic mechanism of arsenic trioxide combination therapy?
Introduction
Arsenic trioxide (As2O3) is the main component of traditional Chinese medicine “Pishuang”, which has an application history of over 2000 years. In the 1970s, Zhang Tingdong from Harbin Medical University, China, uncovered the extraordinary therapeutic effect of arsenic trioxide solution on acute promyelocytic leukemia (APL) (The classification of acute leukemia was shown in Table 1). Since then, the anti-leukemia effect of arsenic trioxide monotherapy was extensively explored, achieving highly complete remission rate in both newly diagnosed and relapsed/refractory APL patients [1,2,3]. In early twenty-first century, arsenic trioxide was approved for the treatment of APL, myelodysplastic syndrome, and multiple myeloma by U.S. Food and Drug Administration (FDA). Nevertheless, therapy with arsenic trioxide may be associated with some side effects, especially cardiotoxicity, which limits its clinical application [4,5,6]. Besides, arsenic trioxide monotherapy has been shown less effective in non-APL acute myeloid leukemia (AML) (lacking t (15;17) translocation) patients [7,8,9]. Therefore, it is important to develop new strategies to promote the therapeutic efficiency of arsenic trioxide and to reduce its toxicity. Combination therapy is a frequently used strategy to alleviate this problem. This review summarizes the research progress of arsenic trioxide combined with anticancer drugs, vitamins and vitamin analogs, plant products and other kind of drugs in the treatment of leukemia, in order to provide new insights for the treatment of leukemia and drug combination research.
Arsenic trioxide combined with anticancer drugs
Cytotoxic anticancer drugs
DNA/RNA synthesis inhibitors
Fludarabine, a nucleotide analog of aryladenosine, has a significant effect on chronic lymphocytic leukemia (CLL) [10]. However, drug resistance is still a problem during fludarabine treatment [11]. CLL cells with chromosome 17p13 deletion are predisposes to fludarabine resistance. Whereas, this subtype is more susceptible to arsenic trioxide. Arsenic trioxide at 2 and 4 μM, preferentially induced apoptosis of B-cell CLL cells from patients with unfavorable prognosis (including those that were resistant to fludarabine), compared to B cells from healthy controls [12]. A further study showed that arsenic trioxide (3 μM) facilitated apoptosis of B-cell CLL cells from patients by the suppression of PI3K/Akt signaling pathway [13]. Arsenic trioxide (1 μM) and fludarabine (5 μM) have a synergistically anti-leukemia effect in untreated and fludarabine-resistant CLL cells from patients. This effect was associated with decreased phosphorylation levels of Akt and ERK, as well as decreased Mcl-1/Bim and Bcl-2/Bax ratios [14]. Therefore, the combination of arsenic trioxide and fludarabine may be an effective therapeutic strategy for overcoming fludarabine resistance in CLL patients.
DNA methyltransferase inhibitors
Decitabine monotherapy or in combination with chemotherapy was effective in refractory and relapsed AML patients [15]. Decitabine and arsenic trioxide could inhibit proliferation of MV-4-11 AML cells (The summary of leukemia cell lines were shown in Table 2) in a concentration dependent manner, with IC50 values of 2.409 and 2.364 μM (2 × 104 cells each well in 96 well-plate, 48 h), respectively. Decitabine (5 μM) and arsenic trioxide (2 μM) play a synergistic effect on inhibiting the proliferation and inducing apoptosis of MV-4-11 AML cells [16].
Anti-cancer antibiotics
Idarubicin is an anthracycline anticancer drug that can be used as a monotherapy in the treatment of acute non-lymphocytic leukemia [17]. In a clinical study, eight relapsed APL patients were enrolled to evaluate the therapeutic efficacy of a single induction course with arsenic trioxide (10 mg/day) followed by consolidation therapy with idarubicin. The combination of idarubicin and arsenic trioxide was effective in achieving molecular remission in relapsed APL patients, and was reducing the long-term toxicity of arsenic trioxide and the mutagenicity of combination chemotherapy [18].
In addition, the increased dose of arsenic trioxide (0.5 mg/kg) in combination with idarubicin and cytarabine was safe and well tolerated in newly diagnosed AML patients. Arsenic trioxide supplementation improved the outcomes of patients compared with those who received cytarabine and idarubicin. Therefore, arsenic trioxide may enhance the efficacy of chemotherapy, which may be related to the downregulation of Stat3 [19].
Protein synthesis inhibitors
Homoharringtonine is an approved anti-leukemia drug, which mainly exist in the plants of Cephalotaxus Sieb. et Zucc. ex Endl. Homoharringtonine (30 ng/ml) and arsenic trioxide (4 μM) could synergistically promote the apoptosis of U-937 AML cells, which was related to the inhibition of PI3K/Akt signaling pathway and its downstream molecule MCL-1 protein expression [20]. A recent case report indicated that the combination of homoharringtonine and arsenic trioxide bring substantial effects on an elderly patient with AML1/ETO [21]. However, the underlying mechanisms still need to be elucidated.
Paclitaxel, an ingredient from taxus chinensis, is one of the most prominent anticancer drugs. However, paclitaxel was not effective in refractory or relapsed acute lymphocytic leukemia (ALL) patients [22]. A synergistic effect of paclitaxel (5 nM) and arsenic trioxide (1 μM) was observed in inhibiting proliferation and inducing apoptosis of Jurkat T-cell ALL cells, which was related to the increased phosphorylation of Cdk1 and formation of the inhibitory spindle checkpoint complex BubR1/Cdc20. In addition, arsenic trioxide also increased the sensitivity to paclitaxel of primary cells from ALL patients [23].
Noncytotoxic anticancer drugs
Tyrosine kinase inhibitors
Tyrosine kinase inhibitors (TKIs) are a common kind of antitumor drugs, which can be divided into multi-targeted and single-targeted TKIs. Clinically, patients with Fms-related tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation are the largest subgroup of AML, which is associated with an increased relapse risk and decreased disease-free survival [24].
Multi-targeted TKI sorafenib has favorable initial outcomes in AML patients, but then is limited due to subsequent drug resistance [25, 26]. Patients with FLT3-ITD mutation are the largest group of AML (~25% of all AML patients) with the poorest prognosis [27]. Arsenic trioxide has relative selective activity on FLT3-ITD cells (MOLM14, MV-4-11, and HB11;19) compared with non-FLT3-ITD leukemia cells (HL-60, SEM-K2, THP-1, U-937, and K-562) [28]. When sorafenib (25 nM) combined with arsenic trioxide (1 μM), a synergistic apoptosis inducing effect was observed in FLT3-ITD MOLM13 AML cells, which is related to the inactivation of FLT3-ITD (both expression and phosphorylation) and GSK3β, the upregulation of Bax and Bak and the downregulation of Mcl-1. Furthermore, the combined treatment also prolonged the survival of mice xenografted with MOLM13 cells [29]. Similarly, the combination of arsenic trioxide (1 and 2 μM) and sorafenib/quizartinib (4 and 8 nM) showed synergistic anti-leukemia effects in FLT3-ITD AML cells (MOLM14 and MV-4-11) and primary cells from patients [28]. In addition, arsenic trioxide and sorafenib combination significantly reduced the viability and promoted the apoptosis of non-FLT3-ITD U-937 cells (arsenic trioxide 1 μM and sorafenib 5 μM) and KG-1 cells (arsenic trioxide 2 μM and sorafenib 7 μM), with an increased transcription of pro-apoptotic and autophagic genes [30]. Therefore, the combination of arsenic trioxide and multi-targeted TKIs (sorafenib and quizartinib) has the potential to improve the outcomes of FLT3-ITD AML patients, which may be also effective in non-FLT3-ITD patients.
Single-target TKIs include imatinib, dasatinib, gefitinib, etc. Imatinib (STI571, a BCR-ABL TKI) is effective in the treatment of chronic myeloid leukemia (CML). Du et al. found that the combination of arsenic trioxide (1 μM) and imatinib (0.25 μM) was more effective and efficient than imatinib monotherapy in inducing apoptosis in K-562 CML cells. They also identified a series of altered genes using microarray analysis, which requires further validation [31]. Nilotinib (a BCR-ABL TKI) (5 nM) and arsenic trioxide (1 μM) in combination inhibited the proliferation but promoted the differentiation of CML cells derived from patients with blast crisis [32]. Arsenic trioxide combined with dasatinib (Src and BCR-ABL inhibitor) synergistically inhibited the proliferation and induced apoptosis of Philadelphia chromosome-positive (Ph+) ALL cells (SUP-B15) and negative ALL cells (TOM-1). This effect was through activating the IRE1/JNK/PUMA axis [33]. Gefitinib (a selective EGFR TKI) (10 μM) could enhance arsenic trioxide (0.6 μM) induced differentiation of NB4 APL cells through promoting ROS production and activation of ERK pathway [34]. The Src family kinase inhibitor PP2 (10 μM) significantly promoted arsenic trioxide (0.5 μM)-induced differentiation of NB4 cells through increasing ICAM-1 and cathepsin D expression [35]. And the triple combination of PP2 (10 μM), ATRA (1 nM) and arsenic trioxide (0.5 μM) enhanced the differentiation of APL cells through increasing ICAM-1 expression [36]. Therefore, the combination of arsenic trioxide and TKIs may be beneficial in the treatment of CML, APL and non-APL AML, especially FLT3-ITD AML.
Protease inhibitors
Bortezomib is a selective and reversible inhibitor of the 26S subunit of the proteasome. Arsenic trioxide (1 μM) and bortezomib (5 nM) synergistically inhibited the proliferation and induced the apoptosis of HL-60 cells through enhancing caspase activation, mitochondrial depolarization, ROS production and abrogating DNA-binding activity of NF-κB. Through microarray analysis, the pathways related to this drug combination (arsenic trioxide 2.2 μM and bortezomib 5.6 nM) were identified, including “proliferation of leukocytes”, “tumorigenesis”, “control of cell cycle”, “hypoxia”, “oxidative stress”, etc. Moreover, in three cases of AML-M2, arsenic trioxide (2.2 μM) and bortezomib (5.6 nM) showed synergistic anti-leukemia activity [37].
AT7519 is a pan-cyclin-dependent kinase inhibitor, which could reduce cyclins expression and induce G1 cell cycle arrest, thereby inhibiting the proliferation of AML KG-1 cells. The combination of AT7519 (0.75 and 1 μM) and arsenic trioxide (2 µM) resulted in a superior cytotoxicity than either drug alone in inhibiting KG-1 cell growth [38]. However, further investigations are still needed.
All-trans retinoic acid
All-trans retinoic acid (ATRA), a metabolic derivative of vitamin A in animals, is efficient in the treatment of APL with a complete remission rate of 90–95% in patients [39]. Unfortunately, relapses frequently accompanied by ATRA resistance, which occur in 15–30% of APL patients [40].
A series of clinical investigations proved the effect of ATRA and arsenic trioxide combined treatment for different condition of patients with leukemia. ATRA combined with arsenic trioxide as first-line treatment can effectively improve the clinical outcomes of newly and relapsed APL [41,42,43,44,45]. Especially, this drug regimen has high efficiency and safety in pediatric patients with APL [46,47,48]. In addition, a recent case report discovered that arsenic trioxide combined with ATRA was effective and safe for a child with down syndrome and APL, who is often intolerable to cytotoxic chemotherapy [49]. This drug combination regime has also proved to be safe and effective for elderly APL patients [50].
Nucleophosmin-1 (NPM1) gene mutation occurs in about 50–60% of AML patients with normal karyotype [51]. The combination of ATRA and conventional chemotherapy selectively improved the survival of AML patients with NPM1 mutation in the absence of FLT3-ITD [52].
In arsenic trioxide (1 μM) and ATRA (1 μM) synergistically play inhibitive effect on OCI-AML3 and primary AML cells harboring an NPM1 mutation, with little effect on non-APL wild type NPM1 AML cells. The combined effect was through inducing proteasomal degradation of mutant NPM1, restored NPM1 nucleolar localization, PML and SUMO-1 nuclear body formation [53]. These findings indicate that combined therapy can further improve the long-term efficacy and safety outcomes of patients.
Studies have revealed the mechanisms of the combined therapy with ATRA and arsenic trioxide. In vitro studies found that ATRA could increase the uptake of arsenic trioxide by leukemia cells through upregulating a transmembrane protein aquaporin-9 (AQP9), which is recognized as a major pathway of arsenic uptake. The expression of AQP9 was reverse correlated with arsenic trioxide sensitivity in leukemia cells. NB4 cells were sensitive, whereas Jurkat, NALM-20, HL-60, and K-562 cells were relatively resistant to arsenic trioxide treatment [54]. The transfection of AQP9 promoted the sensitivity of K-562 cells to arsenic trioxide [54]. ATRA (100 nM) treatment up-regulated AQP9 expression, resulting in increased arsenic uptake in the HL-60 cells [54]. In addition, curcumin could enhance the uptake of arsenic trioxide in NB4 cells by increasing AQP9 expression, which may improve the therapeutic efficiency of arsenic trioxide [55]. These findings suggest that the increasing of AQP9 expression may be a potential strategy to promote the efficiency of arsenic trioxide. Sumi et al. found that co-treatment with arsenic trioxide (0.5 μM) augmented ATRA (0.1 μM) induced HL-60 cell differentiation, which was through the downregulation of PRTN3 expression, and accompanied by a concomitant increase in Sp1 and IL-1β [56]. These findings provide new insights in explaining the synergism between ATRA and arsenic trioxide.
With the development of monoclonal antibody therapeutics, a triple combination with gemtuzumab ozogamycin was developed. Gemtuzumab ozogamycin, an anti-CD33 monoclonal antibody, is the world’s first antibody drug conjugate to be marketed for leukemia. The combination of ATRA and arsenic trioxide, with or without gemtuzumab ozogamycin, is an effective regimen in patients with newly diagnosed APL [57]. Similar results were achieved by a multicenter phase III study [58]. A long-term data confirmed this chemotherapy-free triple combination strategy, which is effective and safe [59]. Besides, a recent study reported that a patient who relapsed from ATRA and arsenic trioxide was successfully treated with gemtuzumab ozogamycin followed by unrelated cord blood transplantation [60].
Subsequently, other triple-drug therapeutic regimens were put forwarded. Danthala et al. modified the drug dosage and proposed an arsenic trioxide, ATRA, and daunorubicin involved strategy for high-risk APL, which achieved durable responses with minimal toxicity [61]. de Almeida et al. found that the combination of gefitinib could mitigate ATRA and arsenic trioxide resistance of APL cells [62]. Devadas et al. proposed a sequential schedule with arsenic trioxide followed by ATRA and daunorubicin, which showed low toxicity, low mortality, and high cure rate [63]. The combination of arsenic trioxide, ATRA, and anthracycline was safe and effective for newly diagnosed APL regardless of FLT3-ITD mutation status [64, 65]. This triple combination regime was also effective for pediatric patients with APL [66].
In addition, tamibarotene (a synthetic retinoid) is from the same family as ATRA, but is more potent and chemically stable than ATRA. Tamibarotene is active in newly diagnosed or relapsed and refractory APL patients, even after chemotherapy, ATRA and/or arsenic trioxide treatment [67, 68]. Clinically, arsenic trioxide combined with tamibarotene achieved a molecular complete remission in a patient with refractory APL, who was insensitive to arsenic trioxide and tamibarotene monotherapy [69]. Therefore, the combination of arsenic trioxide and tamibarotene may be effective and tolerable for refractory APL cases which have no treatment options.
Arsenic trioxide combined with vitamin and vitamin analogs
Vitamin C
Vitamin C, also known as ascorbic acid, is a polyhydroxyl compound that acts as an effective antioxidant. The sensitivity of leukemia cells to arsenic trioxide correlates with intracellular glutathione (GSH) levels. Cells with lower levels of GSH are more sensitive to arsenic trioxide [70, 71]. The arsenic-resistant of cells could be overcome by GSH depletion [72]. Ascorbic acid may improve the efficiency of arsenic troxide by reducing intracellular GSH levels. Ascorbic acid (62.5 μM) combined with arsenic trioxide (1 μM) exert a stronger pro-apoptosis effect compared to single-drug therapy in the HL-60 and SU-DHL-4 AML cells. Whereas, ascorbic acid did not enhance the inhibitory effect of arsenic trioxide on colony formation of normal hematopoietic cells [73]. Vineetha et al. also validated that ascorbic acid can enhance the pro-apoptosis effect of arsenic trioxide on HL-60 cells [74]. In accordance with these findings, Bachleitner-Hofmann et al. found that arsenic trioxide and ascorbic acid have a synergistic effect for patients with AML [75]. While, another clinical study proposed that the combined therapy has limited benefit effect on patients with non-APL AML (relapsed or refractory AML) [76].
Vitamin D
Paricalcitol (19-Nor-1,25(OH)2D2) is a noncalcified vitamin D analog. Studies have found that paricalcitol can effectively inhibit the activity of leukemia cells in bone marrow. Paricalcitol could inhibit the proliferation of HL-60, NB4, and THP-1 leukemia cells [77]. Paricalcitol (0.1 μM for NB4 cells and 0.01 μM for HL-60 cells) combined with arsenic trioxide (0.6 μM for NB4 cells and 0.8 μM for HL-60 cells) inhibited the growth and promoted the differentiation and apoptosis of myeloid leukemia cells. This effect may be due to the decreased expression of PML-RARA fusion protein and the vitamin D metabolizing protein by arsenic trioxide, and thus increased paricalcitol activity [78]. 1,25(OH)2D3 is an activate of vitamin D3. The combination of 1,25(OH)2D3 (50, 100 and 200 nM) could promote arsenic trioxide-induced cell cycle arrest and apoptosis. This combined effect was associated with the activation of p21 and p27, as well as the enhanced expression of vitamin D receptor [79].
Vitamin E
Vitamin E includes eight natural forms. Among them, α-tocopherol is the most abundant and effective one. (+)-α tocopherol succinate (α-TOS), a succinate ester of (+)-α tocopherol, can induce apoptosis of NB4, NB4-R2 (ATRA-resistant) and primary APL cells. A synergistic pro-apoptosis effect was observed in the treatment of NB4 cells when α-TOS (37.68, 75.36 and 150.72 μM) was administered 24 h after arsenic trioxide (1, 2 and 4 μM). On the contrary, coadministration of α-TOS exerted a moderate antagonistic effect on apoptosis induced by arsenic trioxide [80]. For HL-60 cells, coadministration of α-TOS can enhance the pro-apoptosis effect of arsenic trioxide [74]. The strategies that arsenic trioxide combined with vitamin and vitamin analogs still need further investigation, which may improve the clinical outcomes of APL patients with co-morbidities or contraindications to anthracyclines.
Arsenic trioxide combined with plant products
Parthenolide
Parthenolide, an active ingredient from Chrysanthemum morifolium, is a NF-κB inhibitor. It has been suggested that selective NF-κB inhibitors may be usefully in increasing the therapeutic effect of arsenic trioxide [81]. Parthenolide (10 µM) increased cytotoxicity of arsenic trioxide (2.5, 5 and 10 µM) in murine and human leukemia cell lines of myeloid and lymphoid origin, including EL4, Jurkat, K-562, HL-60 cell lines [82]. Similarly, the combination of these two drugs (arsenic trioxide 2 µM and parthenolide 1 µg/ml) decreased the viability and increased G1 phase arrest in MT2 adult T-cell leukemia cells, which was related to the downregulation of CD44, NF-κB, BMI-1 and c-MYC [83]. Besides, the addition of buthionine sulfoximine (an γ-glutamylcysteine synthetase inhibitor) further potentiated the effect of the combined treatment. The effect of the triple drug combination (arsenic trioxide 2 µM, parthenolide 1 µM and buthionine sulfoximine 12.5 µM) was related to the decrease of GSH and ATP levels, and the promotion of oxidative stress in leukemia cell lines. Importantly, healthy donor lymphocytes were largely unaffected by the same treatment regimen [82].
Resveratrol
Resveratrol is a nonflavonoid polyphenol compound. Coadministration of resveratrol substantially amplifies arsenic trioxide inducing autophagy in NB4 cells. The effect of flavonoid genistein was also observed in this project, but less effective than resveratrol [84]. Similarly, another research provided evidence that resveratrol enhances the inhibitory effects of arsenic trioxide on primary AML or CML primitive leukemia progenitors in vitro. Resveratrol (10 and 25 µM) enhanced the pro-apoptotic effect of arsenic trioxide (1 and 2 µM) in KT1 cells, with increased cleaved PARP expression. Low dose of arsenic trioxide (0.5 µM) combined with the same dose of resveratrol could inhibit colony formation in K-562, U-937, KT1 cells and primary cells from CML patients [85]. Further studies proved the combined effect of resveratrol and arsenic trioxide on drug resistant cells. Adriamycin-resistant K-562 leukemia (K-562/RA) cells were cross-resistant to multiple agents (pirarubicin, daunorubicin, 5-FU, etoposide, vincristine and paclitaxel), with the exception of arsenic trioxide. Resveratrol (20 µM) enhanced the proliferation inhibition and apoptosis of K-562/RA and K-562 cells induced by arsenic trioxide (2 µM), which was related to the suppression of drug resistance related genes (P-gp, MRP1 and BCRP) and altered apoptosis-related gene expression [86]. In addition, resveratrol could also alleviates arsenic trioxide-induced cardiotoxicity, hepatotoxicity and nephrotoxicity [87,88,89]. These findings indicate that resveratrol can synergistically enhance the sensitivity of leukemia cells and alleviate the toxicity of arsenic trioxide.
Salvianolic acid A
Salvianolic acid A is an antioxidant exacted from the roots of Salviae miltiorrhizae, which have anticancer and cardioprotective efficiency [90, 91]. The combination of Salvianolic acid A (25 µM) and arsenic trioxide (5 µM) have synergistic activity in K-562 and HL-60 cells [92]. Moreover, Salvianolic acid A could attenuates arsenic trioxide-induced injury in H9c2 cardiomyocytes thorough upregulating Bcl-2 expression and downregulating Bax and Caspase-3 protein expression [93]. Similarly, Salvianolic acid A pretreatment was identified to alleviate arsenic trioxide-induced cardiotoxicity both in vivo and in vitro by improving the damaged mitochondrial function, and the maintenance of normal mitochondrial biogenesis [92]. These findings indicate that the Salvianolic acid A could enhance the anticancer activity of arsenic trioxide and alleviate its cardiotoxicity.
β-Elemene derivative
β-Elemene is an active component of herb medicine Curcuma wenyujin Y.H Chen & C.Ling. It’s derivative N-(β-Elemene-13-yl) tryptophan methyl ester (ETME) (25 µM) alone or in combination with arsenic trioxide (1 µM for NB4 cells and 2 µM for HL-60 cells) can synergistically induces apoptosis in leukemia cells through enhanced production of H2O2, increased expression of cleaved PARP and Caspase-3. Therefore, ETME combined with arsenic trioxide can synergistically induce apoptosis of leukemia cells [94]. These findings indicate that this drug combination may be useful in leukemia patients who do not responsive to arsenic trioxide alone.
Hedyotis diffusa Willd extract
Hedyotis diffusa Willd (HDW) is an annual herb that belongs to the Rubiaceae family. HDW extract can promote the immune response and exhibit inhibitory activity in WEHI-3 leukemia BALB/c mice [95]. A further study validated that HDW alone or combined with arsenic trioxide could promote the total survival rate of BALB/c mice bearing WEHI-3 cells and the inhibitory effect on WEHI-3 cells in a dose-dependent manner. This effect was through enhanced expression of death receptor 4, death receptor 5, Bak and Bid, the activation of Caspase-3, Caspase-8 and Caspase-9, and the inhibition of Bcl-2, Bcl-xl and survivin expression [96]. These studies provide preclinical evidence for the potential efficacy of a combined therapy using HDW with arsenic trioxide in the treatment of APL.
Auraptene
Auraptene is a natural coumarin with anticancer activity [97]. The combination of auraptene and arsenic trioxide synergistically inhibits the viability and induces G1 phase arrest of MT-2 cells through the downregulation of NF-κB (REL-A), CD44, c-MYC, and BMI-1. Therefore, the combined use of auraptene and arsenic trioxide could be considered as a nonchemotherapy regimen for T-cell leukemia [98].
Arsenic trioxide combined with other kinds of drugs
Thalidomide
Thalidomide is a glutamic acid derivative with VEGF inhibitive effect. Mohammadi Kian et al. found that thalidomide combined with arsenic trioxide could synergistically inhibit proliferation, promote apoptosis and autophagy of U-937 (arsenic trioxide 1 µM and thalidomide 60 µM) and KG-1 (arsenic trioxide 1.618 μM and thalidomide 80 μM) AML cells. The effect of combination therapy was better than monotherapy. And the molecular mechanism of combination therapy was related to the downregulation of ULK1 and BECLIN1, and upregulation of PTEN and IL6 [99].
Sulindac
Non-steroidal anti-inflammatory drugs (NSAIDs) have been proven to possess anticancer potential [100]. NSAIDs sulindac sulfide and diclofenac could induce apoptosis and differentiation in THP-1 and HL-60 AML cell lines and primary AML cells from patients [101]. Stepnik et al. found that the combination with sulindac enhanced the cytotoxicity of arsenic trioxide on Jurkat, HL-60, K-562, HPB-ALL and EL4 leukemia cell lines. The combination of arsenic trioxide (1 μM) with sulindac sulfide or sulindac sulfone at concentrations over 50 μM synergistically promoted apoptosis of these cell lines, with little effect on the viability of normal human lymphocytes [102]. Their follow-up study further proved that the cytotoxicity of arsenic trioxide (0.5 or 1 μM) was enhanced by sulindac (100 μM for Jurkat and HL-60 cells, 200 μM for K-562 cells). Interestingly, the metabolites of sulindac (sulindac sulfide and sulindac sulfone) showed higher activity than sulindac. The most effective combination appears to be arsenic trioxide and sulindac sulfide. This combined effect may be due to the induction of apoptosis, but may be not through the induction of ROS production or MAPK pathway activation [103]. Therefore, clinically achievable concentrations of NSAIDs may serve as adjuvant drug for leukemia. However, the combined mechanism still needs to be elucidated.
Buthionine sulfoximine
Buthionine sulfoximine is effective in depleting the cellular level of GSH [72]. Arsenic trioxide (1 μM) and buthionine sulfoximine (10 μM) synergistically induced apoptosis in NB4, U-937, NAMALWA, and Jurkat cells primarily through the activation of c-Jun NH2-terminal kinase (JNK) and upregulation of death receptor 5 and cleaved Caspase-8. Besides, the degradation of IκBα was only observed by combined treatment but not with either agent alone [104]. In addition, buthionine sulfoximine (50 μM) also promoted the susceptibility of HL-60 cells to arsenic trioxide (3 μM). The combined treatment induces mitochondrial injury and apoptosis in HL-60 cells by promoting the phosphorylation of BIMEL and MCL1. Furthermore, the knockdown of BIMEL abolished the effect of the combined treatment [105].
Deferoxamine
Deferoxamine, a hydroxamic acid complexing agent, is an anti-leukemia agent, but its effective dose is relatively high. Therefore, studies to lower the effective dose of deferoxamine were conducted. Yu et al. found deferoxamine combined with arsenic trioxide has synergistic inhibitory effect than monotherapy on HL-60 cells in a nude mouse model, with no significant side effects. The combined effect was associated with the upregulation of Caspase-3 and Bax, and the downregulation of NF-κB p65 and survivin [106].
Rapamycin
The FRAP/mTOR inhibitor rapamycin (also known as sirolimus) is a macrolide immunosuppressive drug. Altman et al. reported that rapamycin (10 nM) enhances the inhibitive effect of arsenic trioxide (1 μM) on primary leukemia progenitors from AML patients [107]. Subsequently, Dembitz et al. found that rapamycin (20 nM) combined with arsenic trioxide (0.5 and 1 μM) has a synergistic antiproliferative and pro-apoptosis effect on AML cell lines (HL-60 and U-937) and primary AML cells that lack typical t (15;17) translocation. This effect may be due to the inhibition of Akt signaling pathway and decrease the expression of anti-apoptotic Mcl-1 protein [108]. The two studies proved the synergistic effect and mechanism of the two drugs at therapeutically achievable doses on non-APL AML cells.
Mannitol
The combination of arsenic trioxide and ATRA achieved a highly curable rate in APL [94]. Nevertheless, extramedullary relapse, commonly in central nervous system, occurs in 3–5% of APL patients [109]. However, intravenous infusion arsenic trioxide was difficult to reach therapeutic level in cerebrospinal fluid [110]. To bridge this gap, a study found that mannitol infusion could increase permeability of blood-brain barrier for arsenic (AsIII), which was beneficial to central nervous system relapsed APL patients [111]. These findings provide useful insights for optimizing treatment outcomes of arsenic trioxide in central nervous system relapsed APL patients.
Venetoclax
Venetoclax (ABT-199) is selective Bcl-2 inhibitor proved by FDA for the treatment of AML. The application of venetoclax showed clinical beneficially for newly diagnosed AML [112]. Venetoclax (200 nM) and arsenic trioxide (3 μM) exert a synergistic effect on inhibiting the viability and promoting apoptosis of KG-1 and KG-1a cells. The combined effect was though inhibiting Akt and ERK, leading to GSK-3β activation and Mcl-1 destabilization [113].
Schiff base oxovanadium complex
Mirjalili et al. found that the IC50 of arsenic trioxide and Schiff base oxovanadium complex on HL-60 cells was 8.37 ± 0.36 μM and 34.12 ± 1.52 μg/ml. Coadministration of extremely low dose arsenic trioxide (0.001 μM) and Schiff base oxovanadium complex (40 μg/ml) could significantly inhibit the cell viability and promote the apoptosis of HL-60 cells, accompanied by the enhanced expression of p21 and p53. Therefore, the combination of these two drugs in the treatment of APL may be with higher efficiency then monotherapy [114].
Oral-arsenic preparation
Oral arsenic trioxide
In 1998, researchers at the University of Hong Kong revived oral arsenic or the “modern” liquor arsenicalis to treat APL patients [115]. The research group from the University of Hong Kong and Queen Mary Hospital did a series of works to observe the clinical effect of oral arsenic trioxide [116]. They proposed that oral arsenic trioxide had a short-term efficacy and safety profile similar to intravenous arsenic trioxide [117]. They then found that oral arsenic trioxide, particularly in prolonged maintenance with and ATRA may obviate the need of stem cells transplantation in relapsed pediatric APL patients [118]. Their recent findings proposed a triple combination regimen with oral arsenic trioxide, ATRA, and ascorbic acid maintenance, which was safe and resulted in favorable long-term survival in APL patients. They are still testing this strategy prospectively to further rigorously assess its long-term effects [119]. Recently, Gill et al. reported the results of a clinical study of newly-diagnosed APL from 1991 to 2021. They found that oral arsenic trioxide-based regimens significantly improved all survivals of APL patients. Therefore, arsenic trioxide (intravenous or oral) should be incorporated into all phases of treatment. In addition, the use of an entirely nonchemotherapy in elderly patients should be explored to reduce drug toxicity [120].
Oral arsenic realgar-Indigo naturalis formula
Another oral arsenic compound, Realgar-Indigo naturalis formula (RIF) with the chemical formula of As4S4, has been shown the highly curative effect for APL treatment, including the adult, pediatric and elderly APL patients.
The researchers from Peking University People’s Hospital did a series of works in comparing the effect of RIF and intravenous arsenic trioxide. They designed a randomized, multicenter, phase III non-inferiority trial to compare the effect of RIF and intravenous arsenic trioxide. They proposed that oral RIF plus ATRA is not inferior to intravenous arsenic trioxide plus ATRA as first-line treatment of APL and may be considered as a routine treatment option for APL patients [121]. They then proposed that oral RIF plus ATRA significantly reduced the medical costs and the length of hospital stay during induction and remission therapy compared with arsenic trioxide plus ATRA in APL patients [122]. A non-inferiority, randomized phase III trial study further validated that RIF plus ATRA is comparable to intravenous arsenic trioxide plus ATRA for non-high-risk APL patients [123]. A subsequent long-term follow-up study further confirmed the effect of this regimen as front-line therapy for non-high-risk APL, and showed that PML-RARA transcript level was associated with relapse [124]. Therefore, this RIF-based chemotherapy-free regimen may be an alternative for non-high-risk APL patients. In addition, they found that RIF and arsenic trioxide showed the similar effects on the recovery of coagulopathy in APL patients [125]. Zhang et al. also validated that RIF application reduces the total hospitalization days and medical costs [126]. This regimen was also reported to be benefit for high-risk APL patients as consolidation therapy [127]. Besides, Li et al. found that sequential application of ATRA, RIF, and chemotherapy shows better efficacy and less toxicity, especially for high-risk patients [128].
Some studies focused on the effect of RIF on pediatric and elderly APL patients. Yang et al. conducted a randomized, multicenter and non-inferiority trial to determine whether intravenous arsenic trioxide can be substituted by oral RIF in the treatment of pediatric APL. They found that RIF is as effective and safe as intravenous arsenic trioxide for the treatment of pediatric APL, with the advantage of reducing hospital stay [129]. Similarly, Luo et al. found that oral RIF can be used as an alternative to intravenous arsenic trioxide for the treatment of pediatric APL patients [130]. Using population pharmacokinetic analysis, Zhang et al. indicated that the AS4S4 formula is safe in newly diagnosed pediatric APL patients [131]. Liao et al. compared the arsenic concentration in plasma and urinary using RIF and intravenous arsenic trioxide, and found that urine arsenic level can estimate plasma arsenic concentration [132]. Lou et al. validated that RIF plus ATRA may be considered as frontline therapy in newly diagnosed APL patients, especially in elderly patients [133]. There is a case report of using RIF and ATRA in a 92-year-old APL patient [50]. Hang et al. also achieved similar results and proposed that the active ingredients in RIF may target ACHE, NCOA2 and RXRA proteins [134]. In addition, Xie et al. investigated the effect of a novel RIF on NB4 cells, and found that it was more effective than RIF in inducing apoptosis of NB4 cells [135].
These findings suggested that oral arsenic-based regimen is safe and effective, and has a substantial reduction in the duration of hospitalization, which helps to improve the patient’s quality of life. In the further, the appropriate chemotherapy-free regimen for high-risk patients and its long-term efficacy is a challenge in the new era.
New approach of arsenic trioxide induced cardiotoxicity
Numerous clinical studies reported that exposure to arsenic trioxide, even at a therapeutic dose, may evoke severe cardiac side effects and even sudden cardiac death in some cases [136,137,138,139]. Therefore, prophylactic measures used to manage the consequent cardiotoxicity in clinical applications of arsenic trioxide are urgently required. It was widely confirmed that arsenic trioxide-induced oxidative stress, apoptosis, and ion homeostasis imbalance are the important causes of cardiotoxicity [87, 140, 141].
The research group in Harbin Medical University is a pioneer of noncoding RNA-involved arsenic trioxide toxicity research. Their previous study revealed the effect of miRNAs in arsenic trioxide-induced cardiotoxicity for the first time. Arsenic trioxide could upregulate miR-1 and miR-133 expression to induce cardiac electrical remodeling in guinea pig myocardium. The knockdown of miR-1 and miR-133 abolished the cardiac electrical disorders caused by arsenic trioxide through upregulation of Kir2.1 and ERG, respectively [142]. Arsenic trioxide could also upregulate miR-21 and miR-23a expression in hERG-HEK293 cells and neonatal rat cardiomyocytes. And the inhibition of miR-21 and miR-23a expression alleviated arsenic trioxide-induced hERG expression deficiency by targeting Sp1 and Hsp90, respectively [143]. We further found that lncRNA Kcnq1ot1 was involved in arsenic trioxide-induced QT interval prolongation [144]. Subsequently, arsenic trioxide was also reported to inhibit lncRNA NEAT1 expression in H9c2 cardiomyocytes, and the overexpression of lncRNA NEAT1 could attenuated the inflammatory response-induced by arsenic trioxide through inhibiting miR-124/NF-κB signaling pathway [145].
The recent study identified differentially expressed mRNAs, miRNAs, lncRNAs and circRNAs in arsenic trioxide-treated mice myocardium, and provided a comprehensive analysis of differentially expressed genes [146]. Noncoding RNAs are potential targets in elimination arsenic trioxide-induced cardiotoxicity [147]. Still, more researches are needed to further validate the exact mechanisms of arsenic trioxide induced cardiotoxicity.
Conclusions
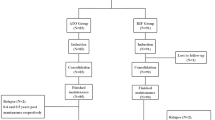
Arsenic trioxide combined therapy is becoming an achievable strategy for the treatment of leukemia (Fig. 1). At present, the combination of arsenic trioxide and anticancer drugs have been used in the treatment of refractory and relapsed leukemia. Other kinds of adjuvant drugs for leukemia are also identified to improve the efficiency of arsenic trioxide, making it available for non-sensitive leukemias. In addition, plant products are more likely to synergistically promote the efficiency of arsenic trioxide and to attenuate its toxicity. However, the mechanisms of combined therapies have not been fully elucidated, which limited the clinical application of these drugs. Noncoding RNAs are expected to become a new mechanism for the arsenic trioxide combined therapy. The present review summarized the current understanding about arsenic trioxide involved drug combination for leukemia (Table 3), in order to provide new insights for the rational use of arsenic trioxide and mechanism study of arsenic trioxide combined treatment for leukemia.
References
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8.
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60.
Shigeno K, Naito K, Sahara N, Kobayashi M, Nakamura S, Fujisawa S, et al. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. Int J Hematol. 2005;82:224–9.
Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, et al. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br J Haematol. 1998;103:1092–5.
Mathews V, Desire S, George B, Lakshmi KM, Rao JG, Viswabandya A, et al. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia. 2006;20:881–3.
Naito K, Kobayashi M, Sahara N, Shigeno K, Nakamura S, Shinjo K, et al. Two cases of acute promyelocytic leukemia complicated by torsade de pointes during arsenic trioxide therapy. Int J Hematol. 2006;83:318–23.
Parmar S, Rundhaugen LM, Boehlke L, Riley M, Nabhan C, Raji A, et al. Phase II trial of arsenic trioxide in relapsed and refractory acute myeloid leukemia, secondary leukemia and/or newly diagnosed patients at least 65 years old. Leuk Res. 2004;28:909–19.
Schiller GJ, Slack J, Hainsworth JD, Mason J, Saleh M, Rizzieri D, et al. Phase II multicenter study of arsenic trioxide in patients with myelodysplastic syndromes. J Clin Oncol. 2006;24:2456–64.
Vey N, Bosly A, Guerci A, Feremans W, Dombret H, Dreyfus F, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006;24:2465–71.
Robertson LE, Denny AW, Huh YO, Plunkett W, Keating MJ, Nelson JA. Natural killer cell activity in chronic lymphocytic leukemia patients treated with fludarabine. Cancer Chemother Pharm. 1996;37:445–50.
Pandzic T, Larsson J, He L, Kundu S, Ban K, Akhtar-Ali M, et al. Transposon mutagenesis reveals fludarabine resistance mechanisms in chronic lymphocytic leukemia. Clin Cancer Res. 2016;22:6217–27.
Merkel O, Heyder C, Asslaber D, Hamacher F, Tinhofer I, Holler C, et al. Arsenic trioxide induces apoptosis preferentially in B-CLL cells of patients with unfavourable prognostic factors including del17p13. J Mol Med. 2008;86:541–52.
Redondo-Munoz J, Escobar-Diaz E, Hernandez Del Cerro M, Pandiella A, Terol MJ, Garcia-Marco JA, et al. Induction of B-chronic lymphocytic leukemia cell apoptosis by arsenic trioxide involves suppression of the phosphoinositide 3-kinase/Akt survival pathway via c-jun-NH2 terminal kinase activation and PTEN upregulation. Clin Cancer Res. 2010;16:4382–91.
Lozano-Santos C, Amigo- Jiménez I, Nova-Gurumeta S, Pérez-Sanz N, García-Pardo A, García-Marco JA. Arsenic trioxide synergistically potentiates the cytotoxic effect of fludarabine in chronic lymphocytic leukemia cells by further inactivating the Akt and ERK signaling pathways. Biochem Biophys Res Commun. 2015;461:243–8.
Ma YY, Zhao M, Liu Y, Zhao DF, Wang LX, Chen XP, et al. Use of decitabine for patients with refractory or relapsed acute myeloid leukemia: a systematic review and meta-analysis. Hematology. 2019;24:507–15.
Chen SS, Zhao YP, Wu WZ, Ma TL, Chen SN. Effect of decitabine in combination with arsenic trioxide on prolife-ration and apoptosis of human acute myeloid leukemia MV4-11 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:1343–7.
Lowenthal RM, Chesterman CN, Griffiths JD, Manoharan A, Harris MG, Herrmann RP, et al. Oral idarubicin as single-agent treatment of acute nonlymphocytic leukemia in poor-risk patients. Cancer Treat Rep. 1987;71:1279–81.
Kwong YL, Au WY, Chim CS, Pang A, Suen C, Liang R. Arsenic trioxide- and idarubicin-induced remissions in relapsed acute promyelocytic leukaemia: clinicopathological and molecular features of a pilot study. Am J Hematol. 2001;66:274–9.
Wetzler M, Andrews C, Ford LA, Tighe S, Barcos M, Sait SNJ, et al. Phase 1 study of arsenic trioxide, high-dose cytarabine, and idarubicin to down-regulate constitutive signal transducer and activator of transcription 3 activity in patients aged <60 years with acute myeloid leukemia. Cancer. 2011;117:4861–8.
Chen P, Jiang X, You PD, Jin Q, Yuan Q, Huang HF. Combination of homoharringtonine with arsenic trioxide induces apoptosis of human acute myeloid leukemia cell line U937. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:1649–53.
He ZP, Chen ML, Huang YP, Chen LL, Wang BX, Wang HX, et al. Acute myeloid leukemia in an 86-year-old man with AML1/ETO treated with homoharringtonine and arsenic trioxide: a case report. Medicine. 2019;98:e14998.
Colburn DE, Thomas DA, Giles FJ. Phase II study of single agent paclitaxel in adult patients with relapsed acute lymphocytic leukemia. Invest N Drugs. 2003;21:109–11.
Duan XF, Wu YL, Xu HZ, Zhao M, Zhuang HY, Wang XD, et al. Synergistic mitosis-arresting effects of arsenic trioxide and paclitaxel on human malignant lymphocytes. Chem Biol Interact. 2010;183:222–30.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9.
Man CH, Fung TK, Ho C, Han HHC, Chow HCH, Ma ACH, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119:5133–43.
Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27:48–55.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
Nagai K, Hou LH, Li L, Nguyen B, Seale T, Shirley C, et al. Combination of ATO with FLT3 TKIs eliminates FLT3/ITD+ leukemia cells through reduced expression of FLT3. Oncotarget. 2018;9:32885–99.
Wang R, Li Y, Gong P, Gabrilove J, Waxman S, Jing YK. Arsenic Trioxide and sorafenib induce synthetic lethality of FLT3-ITD acute myeloid leukemia cells. Mol Cancer Ther. 2018;17:1871–80.
Haghi AT, Salami M, Kian MM, Nikbakht M, Mohammadi S, Chahardouli B, et al. Effects of sorafenib and arsenic trioxide on U937 and KG-1 cell lines: apoptosis or autophagy? Cell J. 2020;22:253–62.
Du YZ, Wang KK, Fang H, Li JM, Xiao DK, Zheng PZ, et al. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood. 2006;107:1582–90.
Wang W, Lv FF, Du Y, Li NN, Chen YL, Chen LH. The effect of nilotinib plus arsenic trioxide on the proliferation and differentiation of primary leukemic cells from patients with chronic myoloid leukemia in blast crisis. Cancer Cell Int. 2015;15:10.
Wang T, Cheng CY, Peng LJ, Gao MQ, Xi MP, Rousseaux S, et al. Combination of arsenic trioxide and dasatinib: a new strategy to treat philadelphia chromosome-positive acute lymphoblastic leukaemia. J Cell Mol Med. 2018;22:1614–26.
Noh EK, Kim H, Park MJ, Baek JH, Park JH, Cha SJ, et al. Gefitinib enhances arsenic trioxide (AS2O3)-induced differentiation of acute promyelocytic leukemia cell line. Leuk Res. 2010;34:1501–5.
Yoon SG, Cheong HJ, Kim SJ, Kim KH, Lee SC, Lee N, et al. Src family kinase inhibitor PP2 has different effects on all-trans-retinoic acid or arsenic trioxide-induced differentiation of an acute promyelocytic leukemia cell line. Cancer Res Treat. 2013;45:126–33.
Jung YS, Cheong HJ, Kim SJ, Kim KH, Lee N, Park HS, et al. Src family kinase inhibitor PP2 enhances differentiation of acute promyelocytic leukemia cell line induced by combination of all-trans-retinoic acid and arsenic trioxide. Leuk Res. 2014;38:977–82.
Canestraro M, Galimberti S, Savli H, Palumbo GA, Tibullo D, Nagy B, et al. Synergistic antiproliferative effect of arsenic trioxide combined with bortezomib in HL60 cell line and primary blasts from patients affected by myeloproliferative disorders. Cancer Genet Cytogenet. 2010;199:110–20.
Zabihi M, Safaroghli-Azar A, Gharehbaghian A, Allahbakhshian Farsani M, Bashash D. CDK blockade using AT7519 suppresses acute myeloid leukemia cell survival through the inhibition of autophagy and intensifies the anti-leukemic effect of arsenic trioxide. Iran J Pharm Res. 2019;18:119–31.
Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–15.
Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–58.
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106:3342–7.
Shepshelovich D, Oniashvili N, Parnes D, Klein A, Muchtar E, Yeshaya J, et al. Acute promyelocytic leukemia with isochromosome 17q and cryptic PML-RARA successfully treated with all-trans retinoic acid and arsenic trioxide. Cancer Genet. 2015;208:575–9.
Efficace F, Platzbecker U, Breccia M, Cottone F, Carluccio P, Salutari P, et al. Long-term quality of life of patients with acute promyelocytic leukemia treated with arsenic trioxide vs chemotherapy. Blood Adv. 2021;5:4370–9.
Kulkarni UP, Selvarajan S, Lionel S, Prakash MA, Palani HK, Balasundaram N, et al. Real world data with concurrent retinoic acid and arsenic trioxide for the treatment of acute promyelocytic leukemia. Blood Cancer J. 2022;12:22.
Autore F, Chiusolo P, Sorà F, Giammarco S, Laurenti L, Innocenti I, et al. Efficacy and tolerability of first line arsenic trioxide in combination with all-trans retinoic acid in patients with acute promyelocytic leukemia: real life experience. Front Oncol. 2021;11:614721.
Li SY, Lu Y, Liu HC, Gang EJ, Le J, Qian SY, et al. Arsenic trioxide and all-trans retinoic acid in the treatment of children with newly diagnosed acute promyelocytic leukemia. Leuk Lymphoma. 2021;62:1267–70.
Zheng HY, Jiang H, Hu SY, Liao N, Shen DY, Tian X, et al. Arsenic combined with all-trans retinoic acid for pediatric acute promyelocytic leukemia: report from the CCLG-APL2016 protocol study. J Clin Oncol. 2021;39:3161–70.
Kutny MA, Alonzo TA, Abla O, Rajpurkar M, Gerbing RB, Wang YC, et al. Assessment of arsenic trioxide and all-trans retinoic acid for the treatment of pediatric acute promyelocytic leukemia: a report from the Children’s Oncology Group AAML1331 Trial. JAMA Oncol. 2022;8:79–87.
Tsumura Y, Yamada Y, Osumi T, Kato M, Terashima K, Shioda Y, et al. Successful treatment with ATRA and arsenic trioxide for a child with down syndrome and acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2020;42:322–5.
Shi XW, Li SY, Tang SH, Lu Y. Successful treatment of acute promyelocytic leukemia in a 92-year-old man using all-trans retinoic acid combined with oral arsenic: a case report. Medicine. 2021;100:e26144.
Akagi T, Ogawa S, Dugas M, Kawamata N, Yamamoto G, Nannya Y, et al. Frequent genomic abnormalities in acute myeloid leukemia/myelodysplastic syndrome with normal karyotype. Haematologica. 2009;94:213–23.
Schlenk RF, Döhner K, Kneba M, Götze K, Hartmann F, Del Valle F, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica. 2009;94:54–60.
Hajj HE, Dassouki Z, Berthier C, Raffoux E, Ades L, Legrand O, et al. Retinoic acid and arsenic trioxide trigger degradation of mutated NPM1, resulting in apoptosis of AML cells. Blood. 2015;125:3447–54.
Leung J, Pang A, Yuen WH, Kwong YL, Tse EWC. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–6.
Han DD, Ma GB, Gao YJ, Su YH. Curcumin synergistically enhances the cytotoxicity of arsenic trioxide in U266 cells by increasing arsenic uptake. Evid Based Complement Altern Med. 2021;2021:3083041.
Sumi D, Suzukawa K, Himeno S. Arsenic trioxide augments all-trans retinoic acid-induced differentiation of HL-60 cells. Life Sci. 2016;149:42–50.
Kozono S, Lin YM, Seo HS, Pinch B, Lian XL, Qiu CX, et al. Arsenic targets Pin1 and cooperates with retinoic acid to inhibit cancer-driving pathways and tumor-initiating cells. Nat Commun. 2018;9:3069.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–305.
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129:1275–83.
Yamada Y, Osumi T, Kato M, Shioda Y, Kiyotani C, Terashima K, et al. Gemtuzumab ozogamicin followed by unrelated cord blood transplantation with reduced-intensity conditioning for a child with refractory acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2022;44:178–80.
Danthala M, Golamari KR, Seshachalam A, Mikkilineni A, Chappidi S, Mekala MB, et al. Walking a tightrope: dosage modifications and treatment outcomes of all-trans-retinoic acid, arsenic trioxide, and daunorubicin for high-risk acute promyelocytic leukemia. JCO Glob Oncol. 2020;6:1749–56.
de Almeida LY, Pereira-Martins DA, Weinhäuser I, Ortiz C, Cândido LA, Lange AP, et al. The combination of gefitinib with ATRA and ATO induces myeloid differentiation in acute promyelocytic leukemia resistant cells. Front Oncol. 2021;11:686445.
Devadas SK, Jain H, Bagal B, Sengar M, Dangi U, Khattry N, et al. Sequential treatment of arsenic trioxide followed by all trans retinoic acid with anthracyclines has excellent long-term cure in acute promyelocytic leukemia. Indian J Hematol Blood Transfus. 2021;37:30–6.
Wu YX, Ke P, Zhou HX, Wu DP, Chen SN, Qiu HY, et al. Safety and efficacy of different doses of anthracyclines combined with arsenic trioxide and all-trans retinoic acid in the treatment of de novo acute promyelocytic leukemia. Hematology. 2021;26:271–6.
Xu LW, Su YZ, Tao HF. All-trans retinoic acid, arsenic trioxide, and anthracycline-based chemotherapy improves outcome in newly diagnosed acute promyelocytic leukemia regardless of FLT3-ITD mutation status. Curr Med Sci. 2021;41:491–7.
Jabbar N, Khayyam N, Arshad U, Maqsood S, Hamid SA, Mansoor N. An outcome analysis of childhood acute promyelocytic leukemia treated with atra and arsenic trioxide, and limited dose anthracycline. Indian J Hematol Blood Transfus. 2021;37:569–75.
Tobita T, Takeshita A, Kitamura K, Ohnishi K, Yanagi M, Hiraoka A, et al. Treatment with a new synthetic retinoid, Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. Blood. 1997;90:967–73.
Sanford D, Lo-Coco F, Sanz MA, Di Bona E, Coutre S, Altman JK, et al. Tamibarotene in patients with acute promyelocytic leukaemia relapsing after treatment with all-trans retinoic acid and arsenic trioxide. Br J Haematol. 2015;171:471–7.
Kojima M, Ogiya D, Ichiki A, Hara R, Amaki J, Kawai H, et al. Refractory acute promyelocytic leukemia successfully treated with combination therapy of arsenic trioxide and tamibarotene: a case report. Leuk Res Rep. 2016;5:11–13.
Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81:796–9.
Zhou L, Jing YK, Styblo M, Chen Z, Waxman S. Glutathione-S-transferase pi inhibits As2O3-induced apoptosis in lymphoma cells: involvement of hydrogen peroxide catabolism. Blood. 2005;105:1198–203.
Davison K, Côté S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–40.
Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–77.
Vineetha RC, Hariharan S, Jaleel A, Chandran M, Nair RH. L-Ascorbic acid and alpha-tocopherol synergistically triggers apoptosis inducing antileukemic effects of arsenic trioxide via oxidative stress in human acute promyelocytic leukemia cells. Front Oncol. 2020;10:65.
Bachleitner-Hofmann T, Gisslinger B, Grumbeck E, Gisslinger H. Arsenic trioxide and ascorbic acid: synergy with potential implications for the treatment of acute myeloid leukaemia? Br J Haematol. 2001;112:783–6.
Aldoss I, Mark L, Vrona J, Ramezani L, Weitz I, Mohrbacher AM, et al. Adding ascorbic acid to arsenic trioxide produces limited benefit in patients with acute myeloid leukemia excluding acute promyelocytic leukemia. Ann Hematol. 2014;93:1839–43.
Kumagai T, O'Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95:896–905.
Kumagai T, Shih LY, Hughes SV, Desmond JC, O'Kelly J, Hewison M, et al. 19-Nor-1,25(OH) 2D2 (a novel, noncalcemic vitamin d analogue), combined with arsenic trioxide, has potent antitumor activity against myeloid leukemia. Cancer Res. 2005;65:2488–97.
Zhang YL, Qiao SK, Guo XN, Ren JH, Zhang JN. Arsenic trioxide-induced cell apoptosis and cell cycle arrest are potentiated by 1,25-dihydroxyvitamin D3 in human leukemia K562 cells. Oncol Lett. 2021;22:509.
Freitas RA, Silva dos Santos GA, Gimenes Teixeira HL, Scheucher PS, Lucena-Araujo AR, Gouveia Lima AS, et al. Apoptosis induction by (+)alpha-tocopheryl succinate in the absence or presence of all-trans retinoic acid and arsenic trioxide in NB4, NB4-R2 and primary APL cells. Leuk Res. 2009;33:958–63.
Tabellini G, Cappellini A, Tazzari PL, Falà F, Billi AM, Manzoli L, et al. Phosphoinositide 3-kinase/Akt involvement in arsenic trioxide resistance of human leukemia cells. J Cell Physiol. 2005;202:623–34.
Duechler M, Stańczyk M, Czyz M, Stepnik M. Potentiation of arsenic trioxide cytotoxicity by Parthenolide and buthionine sulfoximine in murine and human leukemic cells. Cancer Chemother Pharm. 2008;61:727–37.
Kouhpaikar H, Sadeghian MH, Rafatpanah H, Kazemi M, Iranshahi M, Delbari Z, et al. Synergy between parthenolide and arsenic trioxide in adult T-cell leukemia/lymphoma cells in vitro. Iran J Basic Med Sci. 2020;23:616–22.
Fan YH, Chen M, Meng J, Yu L, Tu YF, Wan L, et al. Arsenic trioxide and resveratrol show synergistic anti-leukemia activity and neutralized cardiotoxicity. PLoS ONE. 2014;9:e105890.
Wu EJ, Goussetis DJ, Beauchamp E, Kosciuczuk EM, Altman JK, Eklund EA, et al. Resveratrol enhances the suppressive effects of arsenic trioxide on primitive leukemic progenitors. Cancer Biol Ther. 2014;15:473–8.
Chen J, Tian BY, Zhou CM, Sun JJ, Lin L, Jin SC, et al. A novel resveratrol-arsenic trioxide combination treatment synergistically induces apoptosis of adriamycin-selected drug-resistant leukemia K562 cells. J Cancer. 2019;10:5483–93.
Zhang WQ, Guo CM, Gao RF, Ge M, Zhu YZ, Zhang ZG. The protective role of resveratrol against arsenic trioxide-induced cardiotoxicity. Evid Based Complement Altern Med. 2013;2013:407839.
Zhang WQ, Xue JD, Ge M, Yu ML, Liu L, Zhang ZG. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol. 2013;51:87–92.
Yu ML, Xue JD, Li YJ, Zhang WQ, Ma DX, Liu L, et al. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol. 2013;87:1025–35.
Li XL, Fan JP, Liu JX, Liang LN. Salvianolic acid A protects neonatal cardiomyocytes against hypoxia/reoxygenation-induced injury by preserving mitochondrial function and activating Akt/GSK-3β signals. Chin J Integr Med. 2019;25:23–30.
Pei RZ, Si T, Lu Y, Zhou JX, Jiang L. Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell apoptosis and suppresses tumor growth in acute myeloid leukemia. Leuk Lymphoma. 2018;59:1959–67.
Zhang JY, Wang M, Wang RY, Sun X, Du YY, Ye JX, et al. Salvianolic acid A ameliorates arsenic trioxide-induced cardiotoxicity through decreasing cardiac mitochondrial injury and promotes its anticancer activity. Front Pharm. 2018;9:487.
Zhang JY, Sun GB, Luo Y, Wang M, Wang W, Du YY, et al. Salvianolic acid A protects H9c2 cells from arsenic trioxide-induced injury via inhibition of the MAPK signaling pathway. Cell Physiol Biochem. 2017;41:1957–69.
Yu ZY, Wang R, Xu LY, Dong JH, Jing YK. N-(beta-Elemene-13-yl)tryptophan methyl ester induces apoptosis in human leukemia cells and synergizes with arsenic trioxide through a hydrogen peroxide dependent pathway. Cancer Lett. 2008;269:165–73.
Lin CC, Kuo CL, Lee MH, Hsu SC, Huang AC, Tang NY, et al. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T- and B-cell proliferation in leukemic mice. In Vivo. 2011;25:633–40.
Kuo YJ, Liu YJ, Way TD, Chiang SY, Lin JG, Chung JG. Synergistic inhibition of leukemia WEHI-3 cell growth by arsenic trioxide and Hedyotis diffusa Willd extract in vitro and in vivo. Exp Ther Med. 2017;13:3388–96.
Lee JC, Shin EA, Kim B, Kim BI, Chitsazian-Yazdi M, Iranshahi M, et al. Auraptene induces apoptosis via myeloid cell leukemia 1-mediated activation of caspases in PC3 and DU145 prostate cancer cells. Phytother Res. 2017;31:891–8.
Kazemi M, Kouhpeikar H, Delbari Z, Khodadadi F, Gerayli S, Iranshahi M, et al. Combination of auraptene and arsenic trioxide induces apoptosis and cellular accumulation in the subG1 phase in adult T-cell leukemia cells. Iran J Basic Med Sci. 2021;24:1643–9.
Kian MM, Mohammadi S, Tavallaei M, Chahardouli B, Rostami S, Zahedpanah M, et al. Inhibitory effects of arsenic trioxide and thalidomide on angiogenesis and vascular endothelial growth factor expression in leukemia cells. Asian Pac J Cancer Prev. 2018;19:1127–34.
Wu KK, Na K, Chen D, Wang YJ, Pan HT, Wang XY. Effects of non-steroidal anti-inflammatory drug-activated gene-1 on ganoderma lucidum polysaccharides-induced apoptosis of human prostate cancer PC-3 cells. Int J Oncol. 2018;53:2356–68.
Singh R, Cadeddu RP, Fröbel J, Wilk CM, Bruns I, Zerbini LF, et al. The non-steroidal anti-inflammatory drugs sulindac sulfide and diclofenac induce apoptosis and differentiation in human acute myeloid leukemia cells through an AP-1 dependent pathway. Apoptosis. 2011;16:889–901.
Stępnik M, Ferlińska M, Smok-Pieniążek A, Gradecka-Meesters D, Arkusz J, Stańczyk M. Sulindac and its metabolites: sulindac sulfide and sulindac sulfone enhance cytotoxic effects of arsenic trioxide on leukemic cell lines. Toxicol Vitr. 2011;25:1075–84.
Stępnik M, Ferlińska M, Smok-Pieniążek A, Gradecka-Meesters D, Arkusz J, Stańczyk M. Assessment of the involvement of oxidative stress and Mitogen-Activated Protein Kinase signaling pathways in the cytotoxic effects of arsenic trioxide and its combination with sulindac or its metabolites: sulindac sulfide and sulindac sulfone on human leukemic cell lines. Med Oncol. 2012;29:1161–72.
Chen D, Chan R, Waxman S, Jing YK. Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res. 2006;66:11416–23.
Tanaka Y, Komatsu T, Shigemi H, Yamauchi T, Fujii Y. BIMEL is a key effector molecule in oxidative stress-mediated apoptosis in acute myeloid leukemia cells when combined with arsenic trioxide and buthionine sulfoximine. BMC Cancer. 2014;14:27.
Yu RH, Wang D, Ren XH, Zeng L, Liu YF. The growth-inhibitory and apoptosis-inducing effect of deferoxamine combined with arsenic trioxide on HL-60 xenografts in nude mice. Leuk Res. 2014;38:1085–90.
Altman JK, Yoon P, Katsoulidis E, Kroczynska B, Sassano A, Redig AJ, et al. Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J Biol Chem. 2008;283:1992–2001.
Dembitz V, Lalic H, Ostojic A, Vrhovac R, Banfic H, Visnjic D. The mechanism of synergistic effects of arsenic trioxide and rapamycin in acute myeloid leukemia cell lines lacking typical t(15;17) translocation. Int J Hematol. 2015;102:12–24.
de Botton S, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;20:35–41.
Knipp S, Gattermann N, Schapira M, Käferstein H, Germing U. Arsenic in the cerebrospinal fluid of a patient receiving arsenic trioxide for relapsed acute promyelocytic leukemia with CNS involvement. Leuk Res. 2007;31:1585–7.
Guo MH, Zhao QL, Fan SJ, Wu ZQ, Lin LW, Chen HZ, et al. Characteristics of arsenic species in cerebrospinal fluid (CSF) of acute promyelocytic leukaemia (APL) patients treated with arsenic trioxide plus mannitol. Br J Clin Pharm. 2021;87:4020–6.
Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pr Res Clin Haematol. 2019;32:145–53.
Cho H, Jang JE, Eom JI, Jeung HK, Chung H, Kim JS, et al. Arsenic trioxide synergistically promotes the antileukaemic activity of venetoclax by downregulating Mcl-1 in acute myeloid leukaemia cells. Exp Hematol Oncol. 2021;10:28.
Mirjalili S, Khaleghian A, Kalalinia F. Effects of co-administration of arsenic trioxide and Schiff base oxovanadium complex on the induction of apoptosis in acute promyelocytic leukemia cells. Biometals. 2021;34:1067–80.
Kumana CR, Mak R, Kwong YL, Gill H. Resurrection of oral arsenic trioxide for treating acute promyelocytic leukaemia: a historical account from bedside to bench to bedside. Front Oncol. 2020;10:1294.
Kumana CR, Kwong YL, Gill H. Oral arsenic trioxide for treating acute promyelocytic leukaemia: implications for its worldwide epidemiology and beyond. Front Oncol. 2022;12:1026478.
Au WY, Kumana CR, Kou M, Mak R, Chan GCF, Lam CW, et al. Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. Blood. 2003;102:407–8.
Au WY, Li CK, Lee V, Yuen HL, Yau J, Chan GCF, et al. Oral arsenic trioxide for relapsed acute promyelocytic leukemia in pediatric patients. Pediatr Blood Cancer. 2012;58:630–2.
Gill HS, Yim R, Kumana CR, Tse E, Kwong YL. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: Long-term results and unique prognostic indicators. Cancer. 2020;126:3244–54.
Gill H, Raghupathy R, Lee CYY, Yung Y, Chu HT, Ni MY, et al. Acute promyelocytic leukaemia: population-based study of epidemiology and outcome with ATRA and oral-ATO from 1991 to 2021. BMC Cancer. 2023;23:141.
Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang JX, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215–21.
Jiang H, Liang GW, Huang XJ, Jiang Q, Han S, Shi LW, et al. Reduced medical costs and hospital days when using oral arsenic plus ATRA as the first-line treatment of acute promyelocytic leukemia. Leuk Res. 2015;39:1319–24.
Zhu HH, Wu DP, Du X, Zhang X, Liu L, Ma J, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19:871–9.
Tang FF, Lu SY, Zhao XS, Qin YZ, Liu XH, Jia JS, et al. PML-RARA transcript levels at the end of induction therapy are associated with prognosis in non-high-risk acute promyelocytic leukaemia with all-trans retinoic acid plus arsenic in front-line therapy: long-term follow-up of a single-centre cohort study. Br J Haematol. 2021;195:722–30.
Zhu HH, Guo ZP, Jia JS, Jiang Q, Jiang H, Huang XJ. The impact of oral arsenic and all-trans-retinoic acid on coagulopathy in acute promyelocytic leukemia. Leuk Res. 2018;65:14–19.
Zhang XX, Liu L, Yao YZ, Gong S, Wang MC, Xi JY, et al. Treatment of non-high-risk acute promyelocytic leukemia with realgar-indigo naturalis formula (RIF) and all-trans retinoid acid (ATRA): study protocol for a randomized controlled trial. Trials. 2020;21:7.
Ma YF, Lu Y, Wu Q, Lou YJ, Yang M, Xu JY, et al. Oral arsenic and retinoic acid for high-risk acute promyelocytic leukemia. J Hematol Oncol. 2022;15:148.
Li XX, Wang LQ, Li H, He XP, Li FL, Wang LL, et al. Clinical study on prospective efficacy of all-trans Acid, realgar-indigo naturalis formula combined with chemotherapy as maintenance treatment of acute promyelocytic leukemia. Evid Based Complement Altern Med. 2014;2014:987560.
Yang MH, Wan WQ, Luo JS, Zheng MC, Huang K, Yang LH, et al. Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG-APL clinical study. Am J Hematol. 2018;93:1467–73.
Luo SL, Tian JD, Sun X, Wu FF, Liu Y, Wan WQ, et al. Oral realgar-indigo naturalis formula treatment for acute promyelocytic leukemia in children: a randomized, control clinical trial. Evid Based Complement Altern Med. 2022;2022:8314176.
Zhang L, Yang XM, Chen J, Hu L, Yang F, Zhou Y, et al. Population pharmacokinetics and safety of oral tetra-arsenic tetra-sulfide formula in pediatric acute promyelocytic leukemia. Drug Des Dev Ther. 2021;15:1633–40.
Liao LH, Chen YQ, Huang DP, Wang LN, Ye ZL, Yang LH, et al. The comparison of plasma arsenic concentration and urinary arsenic excretion during treatment with Realgar-Indigo naturalis formula and arsenic trioxide in children with acute promyelocytic leukemia. Cancer Chemother Pharm. 2022;90:45–52.
Lou YJ, Tong HY, Yu WJ, Wei JY, Xu WL, Mao LP, et al. Efficacy and safety of early switching to an outpatient therapy model using oral arsenic plus retinoic acid based-regimen in newly diagnosed acute promyelocytic leukemia. Leuk Res. 2019;83:106168.
Huang QQ, Wang T, Xiong Y, Qu LP, Yin QZ, Zou WJ. Safety and efficacy of Compound Huangdai Tablets combined with all-trans retinoic acid for treatment of acute promyelocytic leukemia: Clinical evidence and potential mechanisms. Chin Herb Med. 2021;14:154–65.
Xie QJ, Yu L, Wang X, Wu ZG, Zhi DJ, Yang J, et al. A novel realgar-indigo naturalis formula more effectively induces apoptosis in NB4 cells. Pak J Pharm Sci. 2019;32:957–62.
Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109:26–29.
Ducas RA, Seftel MD, Ducas J, Seifer C. Monomorphic ventricular tachycardia caused by arsenic trioxide therapy for acute promyelocytic leukaemia. J R Coll Physicians Edinb. 2011;41:117–8.
Mumford JL, Wu KG, Xia YJ, Kwok R, Yang ZH, Foster J, et al. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environ Health Perspect. 2007;115:690–4.
Vizzardi E, Zanini G, Antonioli E, D'Aloia A, Raddino R, Cas LD. QT prolongation: a case of arsenical pericardial and pleural effusion. Cardiovasc Toxicol. 2008;8:41–4.
Zhao XY, Feng TM, Chen H, Shan HL, Zhang Y, Lu YJ, et al. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharm Toxicol. 2008;102:419–25.
Yu XJ, Wang ZY, Shu ZP, Li ZQ, Ning Y, Yun KL, et al. Effect and mechanism of Sorbus pohuashanensis (Hante) Hedl. flavonoids protect against arsenic trioxide-induced cardiotoxicity. Biomed Pharmacother. 2017;88:1–10.
Shan HL, Zhang Y, Cai BZ, Chen X, Fan YH, Yang LL, et al. Upregulation of microRNA-1 and microRNA-133 contributes to arsenic-induced cardiac electrical remodeling. Int J Cardiol. 2013;167:2798–805.
Zhao X, Shi YQ, Yan CC, Feng PF, Wang X, Zhang R, et al. Up-regulation of miR-21 and miR-23a contributes to As2O3-induced hERG channel deficiency. Basic Clin Pharm Toxicol. 2015;116:516–23.
Jiang YN, Du WJ, Chu Q, Qin Y, Tuguzbaeva G, Wang H, et al. Downregulation of long non-coding RNA Kcnq1ot1: an important mechanism of arsenic trioxide-induced long QT syndrome. Cell Physiol Biochem. 2018;45:192–202.
Chen XX, Jiang YJ, Zeng T, Li JJ. Overexpression of the long noncoding RNA NEAT1 protects against As2O3-induced injury of cardiomyocyte by inhibiting the miR-124/NF-kappaB signaling pathway. Eur Rev Med Pharm Sci. 2020;24:1378–90.
Jiang YN, Shen XY, Dong CR, Zhi FN, Gao Y, Shi CP, et al. The whole transcriptome analysis and the circRNA-lncRNA network construction in arsenic trioxide-treated mice myocardium. Biomed Pharmacother. 2022;151:113183.
Shen XY, Zhi FN, Shi CP, Xu JC, Chao YQ, Xu J, et al. The involvement and therapeutic potential of lncRNA Kcnq1ot1/miR-34a-5p/Sirt1 pathway in arsenic trioxide-induced cardiotoxicity. J Transl Med. 2023;21:52.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81803524 and 82170240), Natural Science Foundation of Heilongjiang Province (Grant No. LH2021H018), the Heilongjiang Postdoctoral Foundation (Grant No. LBH-Q21134).
Author information
Authors and Affiliations
Contributions
YJ, BY, and YB contributed to conception of the review. YJ, XS, FZ, JX, and ZW wrote or contributed to the writing of the manuscript. YG drawn the figure.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, Y., Shen, X., Zhi, F. et al. An overview of arsenic trioxide-involved combined treatment algorithms for leukemia: basic concepts and clinical implications. Cell Death Discov. 9, 266 (2023). https://doi.org/10.1038/s41420-023-01558-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01558-z
This article is cited by
-
Mechanisms of genotoxicity and proteotoxicity induced by the metalloids arsenic and antimony
Cellular and Molecular Life Sciences (2023)