Abstract
Pyroptosis is a lytic and inflammatory type of programmed cell death that is mediated by Gasdermin proteins (GSDMs). Attractively, recent evidence indicates that pyroptosis involves in the development of tumors and can serve as a new strategy for cancer treatment. Here, we present a basic knowledge of pyroptosis, and an overview of the expression patterns and roles of GSDMs in breast cancer. In addition, we further summarize the available evidence of pyroptosis in breast cancer progression and give insight into the clinical potential of applying pyroptosis in anticancer strategies for breast cancer. This review will deepen our understanding of the relationship between pyroptosis and breast cancer, and provide a novel potential therapeutic avenue for breast cancer.
Similar content being viewed by others
Facts
-
Pyroptosis is an inflammatory type of programmed cell death that is mediated by Gasdermin proteins (GSDMs).
-
GSDMs are abnormally expressed in breast cancer and are involved in cancer progression.
-
Pyroptosis is associated with inflammatory cytokine production, which exerts effects on cancer progression and tumor microenvironment.
-
Pyroptosis can be triggered by chemotherapy, radiotherapy, nanomaterials, and several small-molecule chemicals or medications.
-
Triggering pyroptosis combined with immunotherapy provides a novel therapeutic potential for breast cancer treatment.
Open questions
-
Does the occurrence of pyroptosis increase or decrease the risk of breast cancer?
-
What is the exact mechanism for pyroptosis to activate the immune response?
-
Are there additional pyroptosis inducers and regulatory genes that remain to be discovered?
-
How to reduce the side effects of pyroptosis inducers when applied clinically?
Introduction
Breast cancer is the leading cause of cancer morbidity and mortality in women around the world [1]. Despite recent advances in breast cancer treatment, there are still a considerable number of patients who acquire drug resistance after systemic treatments such as endocrine therapy, chemotherapy, and targeted therapy, which presents a dilemma for further therapeutic intervention [2, 3]. With the in-depth research on the mechanism of tumor drug resistance, resistance to programmed cell death is one of the recognized mechanisms of tumor drug resistance [4]. Therefore, the induction of programmed cell death (PCD) in tumor cells is expected to be a significant advance in reversing drug resistance and is predicted to serve as the foundation of translational medicine in the future [5,6,7].
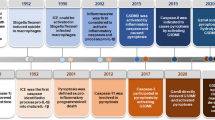
As a form of pro-inflammatory programmed cell death, pyroptosis differs from other types of programmed cell death, such as apoptosis, autophagy, and ferroptosis in terms of its morphology, biochemistry, and molecular process. In the 1990s, researchers revealed for the first time that Shigella flexneri and Salmonella typhimurium could trigger the lytic death of macrophages [8, 9]. Subsequent studies found that this type of cell death is dependent on caspase-1 activation, which is different from the traditional form of apoptosis that depends on the activation of caspase-3, and named it pyroptosis for the first time [10, 11]. Even though the fact that the concept of pyroptosis was introduced relatively early in the research process, it is challenging to make a breakthrough in the study of its exact occurrence process and molecular mechanism, resulting in relatively slow progress in the initial stages of pyroptosis research. Until recently, researchers discovered that the Gasdermin family proteins are the key execution molecules of pyroptosis [12,13,14,15], which makes the study of pyroptosis an attractive topic for cancer research.
Emerging evidence indicates that pyroptotic cell death leads to tumor growth suppression and targeting pyroptosis might be a promising anticancer strategy. Recent findings of new approaches to trigger pyroptosis in breast cancer cells and further identified the function of pyroptosis-related genes in breast cancer progression serve as a foundation for developing strategies for targeting pyroptosis in breast cancer treatment. In this review, we first present a current overview of the expression patterns and roles of pyroptosis-related molecules in breast cancer and discuss the potential impact of pyroptosis on the development and treatment of breast cancer.
Mechanisms of pyroptosis
Canonical pathway
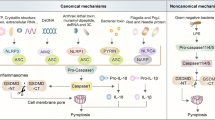
The canonical pathway, which is dependent on caspase-1 activation, was the first pyroptosis pathway to be identified. When cells respond to various pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), cytosolic pattern recognition receptors (PRRs) are activated, thereby stimulating cells to form corresponding inflammasomes (NLRP1, NLRP3, NLRC4, AIM2, and PYRIN inflammasomes) [16, 17]. In addition, the inflammasome is responsible for the activation of the caspase-1 protein by recruiting an Apoptosis-associated speck-like protein containing a CARD (ASC) and procaspase-1 protein. On the one hand, the activated caspase-1 protein has the ability to cleave the GSDMD protein, release an N-terminal domain of GSDMD (N-GSDMD), and initiate pyroptosis in cells [12, 18]. On the other hand, it can cleave the precursors of IL-1β/IL-18 into mature IL-1β/IL-18 cytokines, which are then secreted outside the cells to further amplify the process of inflammatory signals and cell pyroptosis [19].
Non-canonical pathway
The non-canonical pyroptosis pathway is mediated by the caspase proteins caspase-4/5/11, with caspase-4/5 being present in humans and caspase-11 being present in mice [20]. Bacterial lipopolysaccharide (LPS) can directly bind and activate caspase-4/5/11 and further cleave GSDMD into N-GSDMD, thereby initiating the pyroptosis program [13, 21, 22]. Unlike caspase-1, caspase-4/5/11 is incapable of cleaving pro-IL-1β/pro-IL-18. However, the non-canonical pyroptosis process can also activate the NLPR3/caspase-1 pathway, which ultimately results in the maturation and secretion of IL-1β/IL-18 [23]. Besides, N-GSDMD cleaved by caspase-4/5/11 leads to a drop in intracellular potassium levels, which could activate NLRP3 inflammasome as well [24]. In addition, cleaved caspase-11 could activate the pannexin-1 channel to induce ATP release, which in turn activates the purinergic P2X7 receptor to mediate pyroptosis [25].
Other pathways
It was reported that several chemotherapeutic agents can induce pyroptosis in tumor cells via the caspase-3/GSDME pathway [26]. In addition, caspase-8 in tumor cells can be activated by TNF-α to cleave GSDMC [27], while in murine macrophage, activation of caspase-8 in the context of TAK1 inhibition results in cleavage of both GSDMD and GSDME [28].
Interestingly, Granzyme A (Gzm A), which is secreted by cytotoxic T lymphocytes and natural killer cells, enters target cells with the assistance of perforin and could directly cleave GSDMB, resulting in cell pyroptosis [29]. Moreover, Granzyme B (Gzm B) can cleave GSDME in an indirect manner by activating caspase-3, or it can also directly cleave GSDME to initiate pyroptosis [30], which provides a new therapeutic strategy for future tumor immunotherapy. Recently, through a genome-wide CRISPR-Cas9 system, Deng et al. successfully identified streptococcal pyrogenic exotoxin B (SPEB) that can cleave GSDMA and lead to pyroptosis [31], which further enriches and extends the understanding of pyroptosis. The main pathways involved in pyroptosis are summarized and presented in Fig. 1
A Canonical pathway. In the canonical pathway, pathogens lead to inflammasome formation and activation of caspase-1, which subsequently cleaves GSDMD and promotes IL-1β/IL-18 maturation. GSDMD-N-terminal domain forms pores in the plasma membrane, further resulting in cell lysis and IL-1β/IL-18 release. B In the noncanonical pathway, caspase-4/5/11 is activated by cytosolic LPS, triggering pyroptosis by cleaving GSDMD. C Yersinia, TNF-αsignaling, Gzm B, and chemotherapeutic drugs involve in the activation of caspase-8 and caspase-3, which induce pyroptosis through cleavage of GSDMC, GSDMD, or GSDME. In addition, Gzm A, Gzm B, and SPEB also cause pyroptosis by directly cleaving GSDMB, GSDME, and GSDMA, respectively.
Gsdms in breast cancer
The GSDM superfamily contains six genes (GSDMA to E and DFNB59) in humans [32]. Except for the PJVK protein, other GSDMs proteins feature an N-terminal domain (with cell membrane hole punching activity), a C-terminal domain (with inhibitory activity), and a hinge region structure connected in the middle [33]. Given that the GSDMs execute the ultimate function in the process of pyroptosis, scientists redefine pyroptosis as a form of Gasdermin-mediated programmed cell death [14]. Therefore, we provide an overview of GSDMs in breast cancer, with a focus on their expression profiles, regulation mechanism, and biological characteristics involved in cancer biology (Table 1).
GSDMA
Humans contain a single gene for GSDMA, while mice have three homologs of Gsdmas (Gsdma1–3) [33, 34]. Human GSDMA is primarily detected in normal epithelial cells but is generally depleted in cancer cells [35]. In 2000, Saeki et al. first detected the mRNA expression of GSDMA in eight breast cancer cell lines and observed no transcript of GSDMA in any of the cell lines, even with the amplification of the GSDMA gene [36]. However, treatment with 5-aza-2’-deoxycytidine (5-aza-dC), a DNA methyltransferase inhibitor, could upregulate the expression of GSDMA but not GSDMB in the MCF7 cell [37], indicating that GSDMA expression is mediated by DNA methylation in breast cancer. Since there have been only a few studies on GSDMA in breast cancer, further studies are needed for a deeper understanding of the role of GSDMA in breast cancer.
GSDMB
Although GSDMA and GSDMB are located at the same human chromosomal region (17q21) [38], unlike GSDMA, GSDMB is markedly over-expressed in breast cancer and acts as an oncogene [39, 40]. Interestingly, research had shown that there are four isoforms of GSDMB and different isoforms play different roles in breast cancer. In 2014, Hergueta et al. compared four variants of GSDMB level in breast cancer samples as well as normal mammary tissue and reported that only isoform 2 (GSDMB-2) was significantly up-regulated in breast cancer. Furthermore, they also documented that both GSDMB-1 and GSDMB-2 could enhance breast cancer cell metastasis in a specific signaling pathway [39]. These data indicated different splice variants of GSDMB might play a complex role in breast cancer progression and the potential mechanism has not been fully identified.
When referred to different molecular types of breast cancer, GSDMB is found to be intensively amplified in HER2-positive breast cancer and is related to poor prognosis. Moreover, over-expressed GSDMB contributes to resistance to chemotherapy and anti-HER2 therapies in HER2-positive breast cancer patients [40]. Based on the above results, researchers developed a new biocompatible nanocarrier, which works as a vehicle for intracellular delivery of an anti-GSDMB antibody into HER2 breast cancer cells. Intriguingly, targeted anti-GSDMB nanotherapy effectively restricted HER2 breast cancer cell aggressiveness and specifically increased sensitivity to trastuzumab [41]. Therefore, targeting GSDMB might serve as a promising strategy against HER2-positive breast cancer in the future.
GSDMC
The aberrant over-expression of GSDMC was initially identified in metastatic melanoma cells in mice [42], suggesting that GSDMC could promote tumor progression. However, several studies had revealed that the expression profile and biological function of GSDMC in different tumor tissues are inconsistent [43,44,45]. In breast cancer, Xu et al. compared the expression level of GSDMC in breast cancer cells and normal breast cells (MCF10A) and proposed that GSDMC was markedly up-regulated in breast cancer cells [46]. Recently, Hou et al. reported that hypoxia stress and certain chemotherapeutic drugs could activate GSDMC expression. Mechanistically, p-Stat3 could physically bind with nuclear-translocated PD-L1 and its complexation interacts with the site of the GSDMC promoter, enhancing GSDMC expression transcriptionally in breast cancer cells [27]. Nevertheless, the specific functions of GSDMC in breast progression are still unclear.
GSDMD
Human GSDMD is widely expressed in epithelial cells, immune cells, and certain cancers [45, 47,48,49,50]. A previous study conducted immunohistochemical analysis on 108 cases of breast cancer tissues and 23 cases of para-cancerous normal specimens [51]. The expression level of GSDMD in breast cancers was significantly lower than that in neighboring normal tissues. In addition, breast cancer patients with high expression of GSDMD have lower tumor clinical stage and histological grade. Likewise, the higher expression of GSDMD was related to better prognosis in breast cancer patients. These results imply that GSDMD acts as a tumor suppressor in breast cancer progression. However, the underlying mechanism remains obscure. In terms of triple-negative breast cancers, Yan et al. detected that the expression of GSDMD was remarkably upregulated after cisplatin-based neoadjuvant chemotherapy [52]. This finding might provide a new strategy for overcoming cisplatin-resistant breast cancers.
GSDME
As reported, GSDME expression was frequently epigenetic silenced by methylation in several cancers [53,54,55,56], including breast cancers. In 2008, Kim et al. identified that the mRNA level of GSDME was significantly down-regulated in primary breast cancers compared to normal tissues [57]. However, 5-aza-dC could trigger GSDME expression at the transcription level in breast cancer cells, and a quantitative DNA methylation assay further proved the promoter hypermethylation of GSDME [57, 58]. Interestingly, the expression level of GSDME was markedly higher in the ER-negative compared with ER-positive breast cancers [59, 60]. As mentioned above, although GSDME expression is regulated by methylation, the relationship between ER status and GSDME methylation is still ambiguous and requires further studies [57, 60, 61].
Similarly, another study also analyzed the GSDME promoter methylation in a larger number of breast cancer samples and proposed that GSDME methylation was a potential biomarker for breast cancer diagnosis [61]. Moreover, based on the public database, researchers further investigated all 22 CpGs in the GSDME gene and combined two CpGs (one in the gene body, another in the gene promoter) to build a strong predictive model for detecting breast cancers [60]. In addition, survival analysis revealed that GSDME gene body methylation was significantly associated with patient prognosis [60]. Taken together, these findings indicate that GSDME methylation presents a suitable biomarker for breast cancer detection and prognosis.
In terms of the biological function of GSDME, Kim et al. conducted cell proliferation and invasion assays in vitro and revealed that GSDME acts as a tumor suppressor [57]. Consistently, Wang et al. also identified that CDK7 inhibition induced the expression of GSDME in a p53-dependent pathway, thus inhibiting breast cancer cell growth and promoting cell death [62].
Pyroptosis in breast cancer progression
PCD plays multifunctional roles in breast cancer biological processes [63,64,65]. Relevant studies had verified that dysregulation of autophagy is associated with breast cancer pathogenesis and metastasis [66, 67]. Ferroptosis, which is characterized by intracellular iron accumulation and lipid peroxidation, has implications for the progression of breast cancers as well [68]. In terms of pyroptosis, emerging evidence also shows that pyroptosis participates in the physiological and pathological processes of breast cancer. As stated above, although pyroptosis-related genes could affect breast cancer progression, we here focus on the impact of pyroptosis outcomes in the development of breast cancer.
Proliferation and invasion
Activation of tumor cell pyroptosis leads to lytic death of tumor cells, which directly inhibits breast cancer growth and proliferation [69, 70]. Therefore, inducing pyroptosis might be a novel therapeutic strategy for breast cancer. However, as pro-inflammatory programmed cell death, pyroptosis leads to the rupture of the cell membrane and the release of some intracellular cytokines, such as IL-1β and IL-18 [15]. Notably, the secreted IL-1β/IL-18 activates downstream signaling pathways by binding to their respective receptors (IL1R1, IL-18Rα, and IL-18Rβ) on tumor cells, therapy performing multiple functions in tumor progression.
It was reported that an increased level of IL-1β in serum was identified in breast cancer patients and related to an advanced stage and poor prognosis [71,72,73]. Meanwhile, increasing evidence had revealed that IL-1β promotes the proliferation and metastasis of breast cancer cells in vitro and in vivo [73,74,75,76,77,78,79,80]. Jang et al. revealed that IL-1β up-regulated KI-67 expression and accelerated cell cycle in breast cancer cell lines [73]. More specifically, IL-1β could directly promote epithelial–mesenchymal transition [76, 81], stimulate tumor cell adhesion to endothelial cells [82], activate matrix metalloproteinase (MMP) secretion [83], and mediate related-malignancy signaling pathways [84, 85], leading to pathological progression of breast cancers. Although mounting studies confirmed the role of IL-1β in promoting tumor progression, the opposite function of IL-1β has also been proposed. Voloshin et al. demonstrated that recombinant IL-1β does not directly affect the invasive properties of breast cancer cells in vitro [86]. At the same time, a recent finding addressed that IL-1β maintains metastasis-initiating cells (MICs) in a high mesenchymal state, which facilitates MICs invasion but prevents colonization [87], implicating that IL-1β functions both pro- and anti-tumor effects in breast cancer metastasis.
Besides, an increased serum IL-18 level was observed in the advanced-stage of breast cancer patients as well [88, 89], which was consistent with IL-1β. Furthermore, there is evidence that IL-18 promotes breast cancer cell invasion and migration [90] and is associated with the suppression of claudin-12 expression and activation of the p38–MAPK pathway [91].
Hence, pyroptotic cell death in breast cancer cells results in lytic cell death on the one hand, whereas cytokines generated during pyroptosis can also confer beneficial effects on tumorigenesis and cancer progression.
Tumor microenvironment (TME)
TME involves primary tumor cells, various stromal and immune cells, extracellular matrix, cytokines, etc. [92]. There is emerging interest in an improved understanding of pyroptosis in dynamically regulating TME, thus modulating tumor progression and treatment applications [93,94,95].
Accumulating evidence indicates that pyroptosis of tumor cells can result in the reorganization of the immune microenvironment and activate tumor immunity. Zhang et al. demonstrated that Granzyme B could induce pyroptosis in GSDME-positive breast cancer cells, which, in turn, improves tumor-associated macrophage phagocytosis and tumor-infiltrating lymphocytes (CD8+ T and NK cells) counts and activities [30]. As previously stated, GSDME was generally epigenetic silenced by methylation, whereas decitabine increased GSDME expression by blocking DNA methylation. Zhao et al. constructed a biomimetic nanoparticle (BNP) loaded with indocyanine green (ICG) and decitabine, which trigger breast cancer cell pyroptosis by stimulating caspase-3 cleavage to GSDME. In this experiment, tumor pyroptosis increases the concentration of inflammatory factors (IL-6 and TNF-α) in mouse serum, as well as dendritic cell maturation and CD4+ and CD8+ cells infiltration in the TME [96]. Intriguingly, an exciting result by Wang et al. revealed that only a small percentage of tumor cells undergo pyroptosis, but the entire 4T1 mammary tumor graft is eradicated in vivo [97]. Further immune-cell-subtype analysis conducted by single-cell RNA sequencing showed that tumor cell pyroptosis significantly boosted the populations of CD4+, CD8+, NK, and M1 macrophage cells in tumor immune microenvironment, thus leading to a powerful immunogenic response in cancer. As a result, the recruited cytotoxic lymphocytes could further promote tumor cell pyroptosis by releasing granzymes, thereby developing a positive feedback system [29, 30].
Moreover, inflammatory cytokines and various DAMPs released during tumor pyroptosis are also key players in TME. Infiltration of myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), T helper IL-17-producing cells (Th17s), T-regulatory cells (Tregs), and CAFs were reported as immunosuppression [98]. Several studies revealed that IL-1β exerts dual effects in TME by regulating different immune cells. On the one hand, IL-1β-deficient was associated with the decreased infiltration of MDSCs [99] and increased CD8+ cytotoxic T cells [100], thus activating tumor immunity in breast cancer. On the other hand, targeting IL-1β could also promote TAM polarization toward the M2 phenotype, which results in breast cancer metastasis [86]. Like IL-1β, IL-18 has both pro and antitumorigenic effects by regulating the TME in breast cancer as well. Park et al. demonstrated that tumor-derived IL-18 increased immunosuppressive CD56dim CD16dim/− NK cell fraction and induced PD-1 expression in these NK cells, leading to a bad prognosis in TNBC patients [101]. Nevertheless, IL-18 also promotes IFN-γ production in Th1 cells and NK cells, thus enhancing the anti-tumor ability of CD8+ cells [102].
Along with inflammatory cytokines, several DAMPs are also released during the pyroptosis of cancer cells. The high mobility group box 1 protein (HMGB1) acts as a DNA-binding protein and physiologically locates it in the nucleus. Once released during the pyroptosis, HMGB1 binds to toll-like receptor (TLR) 2/4 on the surface of immune cells, which activates transcription factors NF-κB and triggers an innate immune response by releasing TNF-α and IL-6 [103]. In addition to TLR 2/4 receptors, HMGB1 could also activate the NF-κβ and ERK1/2 pathway by interacting with the receptor for advanced glycation endproducts (RAGE) [104]. Other DAMPs, such as ATP and heat shock proteins (HSPs), bind to the corresponding receptors on the surface of antigen-presenting cells (APCs), thereby activating the adaptive immune response [105, 106].
Taken together, pyroptosis of breast cancer cells exerts a crucial role in modulating the TME and exhibits both anti-tumor and pro-tumor immunity. Therefore, in the future, deeper insights into the effects of pyroptosis in regulating TME should be delivered in greater detail.
Pyroptosis in breast cancer therapy
Chemotherapy
Chemotherapy plays an important role in the systemic treatment of breast cancer. According to the results of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, systemic adjuvant chemotherapy reduces the 10-year mortality of breast cancer patients by one-third [107]. Recently, several published studies have shown that conventional chemotherapeutic drugs can induce pyroptosis in breast cancer.
Yan et al. uncovered that cisplatin induces pyroptosis in TNBC in vitro and in vivo through NLRP3/caspase-1/GSDMD pyroptosis pathway [52]. Hou et al. treated TNBC cell line MDAMB231 with various chemotherapy drugs and discovered that pyroptosis was only observed with types of antibiotics (daunorubicin, doxorubicin, epirubicin, and actinomycin D) by activating nPD-L1/GSDMC pathway [27]. Similarly, Zhang et al. applied paclitaxel, cisplatin, doxorubicin, cyclophosphamide, and 5‐fluorouracil to MDAMB231 and T47D cell lines and observed that doxorubicin triggers typical pyroptotic morphology in both cell lines. Furthermore, doxorubicin treatments induced ROS accumulation, which stimulated the phosphorylation of JNK, and induces pyroptosis via the caspase3/GSDME pathway [108]. However, as mentioned above, the GSDME is regulated by methylation in breast cancer, which heavily limits the induction of pyroptosis by chemotherapeutic drugs through GSDME protein cleavage. Fan et al. develop a strategy of combining decitabine (DAC) with chemotherapeutic nanocarriers. As a result, DAC was performed to up-regulate the expression of GSDME through demethylation, thus enhancing the efficacy of chemotherapeutic agents in inducing pyroptosis [109]. Recently, Li et al. presented a carrier-free chemo-photodynamic nanoplatform (assemble cytarabine with chlorin e6) that could effectively triggering pyroptosis in breast cancer as well [110].
Immunotherapy
Immunotherapies include immune checkpoint inhibitors (ICIs), tumor vaccines, monoclonal antibodies, and adoptive immune cell therapies. Notably, ICI is a promising therapeutic strategy for some cancer types (such as non-small cell lung cancer or melanoma) and brings hope to breast cancer, especially for TNBC. Compared to other types of breast cancer, TNBC is considered a good candidate for ICIs because of the relatively higher tumor mutational burden (TMB) and higher tumor-infiltrating lymphocytes (TILs) density [111, 112]. However, in the field of TNBC immunotherapy, the efficacy of ICIs, when used as monotherapies, is not satisfactory [113]. Therefore, improving the efficacy of immunotherapy for TNBC is expected to significantly improve the prognosis of patients and is a great issue in current cancer research.
The predictive role of pyroptosis on the response to breast cancer immunotherapy remains elusive. On the one hand, the induction of pyroptosis of lymphocytes around tumors will help immune evasion, and on the other hand, pyroptosis can also activate anti-tumor immunity. In breast cancer, hypoxia-induced upregulation of intracellular and extracellular gp96, which induces CD8+ T cell pyroptosis through the GSDMD-dependent pathway and facilitates immune evasion [114]. As mentioned above, pyroptosis of tumor cells and the release of inflammatory cytokines result in the reorganization of TME, which will help to improve the response rate of tumor immunotherapy. In a mouse breast cancer model, the researchers coupled GSDMA3 protein to nanoparticles and further delivered it into mouse tumor tissue. The results delivered that although <15% of tumors suffer from pyroptosis, a large number of inflammatory factors and activated cytotoxic T cells released during pyroptosis subsequently, thereby amplifying the anti-tumor immune response and significantly sensitizes 4T1 tumors to anti-PD1 therapy [97]. Similarly, Su et al. applied oncolytic viruses with inhibitor nanoprodrugs MPNPs to synergistically ignite GSDME-mediated pyroptosis, thus reversing an immunosuppressive tumor microenvironment and increasing the efficacy of anti-PD-1 therapy [115].
In addition to regulating tumor immunity through pyroptosis, approaches that activate anticancer immunity and pyroptosis at the same time are of increasing interest. In a mouse model of breast cancer, Zhang et al. showed that the TME of Gsdme−/− mice was associated with fewer TILs and TAMs [30]. Therefore, overexpression of endogenous GSDME in breast cancer is expected to simultaneously activate pyroptosis and promote immune cell infiltration, thus effectively suppressing tumor growth. Elion et al. proved that RIG-I activation induces pyroptosis in breast cancer cells through the caspase-1/GSDMD canonical pathway. Meanwhile, RIG-I agonist activates innate immunity and increases breast TILs in TME, and the combination of RIG-I agonist with anti-PD1 therapy significantly suppressed tumor development to a greater extent than either agent alone in vivo [116]. Taken together, pyroptosis might elicit additive or synergistic effects of immunotherapy for breast cancer.
In clinical practice, the efficacy of immune checkpoint inhibitors (ICIs), when used as monotherapies, is not satisfactory [113, 117], and the oncologist tends to combine ICIs with chemotherapeutic regimens for breast cancer treatment [118]. Nonetheless, it is worthwhile to investigate which chemotherapeutic medicines are most effective when combined with ICIs. In the TONIC trial, metastatic TNBC patients were randomized to nivolumab with different methods of induction therapies, consisting of irradiation, cyclophosphamide, cisplatin, or doxorubicin [119]. The results revealed that the objective response rate (ORR) was 20% for all patients and the majority of ORR was observed in doxorubicin cohorts. Moreover, three phase-III clinical trials (KEYNOTE522 [120], IMpassion031 [121], and NeoTRIP [122]) evaluated the potential efficacy of ICIs with neoadjuvant chemotherapy regimens in early-stage TNBC patients. Unlike KEYNOTE522 and IMpassion031 trials, patients involved in the NeoTRIP trial were not administered doxorubicin or epirubicin treatment. However, in the NeoTRIP trial, the combination of atezolizumab with nab-paclitaxel and carboplatin did not significantly increase the proportion of pathological complete remission (pCR) in TNBC patients, compared to no atezolizumab patients (OR 1.18; 95% CI 0.74–1.89; P = 0.48) [122]. Since in vitro tests have shown that anthracyclines can cause pyroptosis in breast cancer cells [27, 108], we hypothesized if these improvements in ICIs treatment would be attributable to anthracycline-induced pyroptosis in breast cancer cells. Therefore, a thorough knowledge of the molecular mechanism and process of chemotherapeutic drugs-induced pyroptosis in breast cancer cells can assist locate more suitable therapeutic adjuvants in breast cancer immunotherapy, thereby strengthening the efficiency of ICIs for breast cancer patients.
Other therapies
Although pyroptosis has been studied extensively in chemotherapy and immunotherapy, its effect on the endocrine treatment and radiation for breast cancer is still ambiguous. Estrogen receptor alpha (ERa), a transcription factor encoded in the ESR1 gene, is a positive prognostic factor for endocrine therapy in breast cancer. In vitro, IL-1β induces down-regulation of ERa expression by inducing methylation of the ESR1 promoter, thus promoting tamoxifen resistance in hormone receptor-positive breast cancer cells [123]. Pham et al. treated breast cancer cells with different three inflammasome inhibitors that suppress inflammasome activity at distinct phases [74]. According to the results, treatment with inflammasome inhibitors appeared to inhibit the proliferation of breast cancer cells in estrogen receptor-positive breast cancer cells (MCF7 and T47D), but this effect was not observed in triple-negative breast cancer cells (MDAMB231). These findings prompted researchers to further investigate the relationship between the estrogen receptor signaling pathway and the pyroptosis system in breast cancer cells.
Radiotherapy is another major treatment for breast cancer. Cao et al. represented that irradiation could induce pyroptosis in GSDME high-expressing tumor cells and could effectively promote the infiltration of CD8+ T lymphocytes [124], which provides a novel therapeutic strategy for the effects of radiotherapy. In addition, as a novel tumor ablation technique, high-frequency irreversible electroporation (H-FIRE) eliminates breast cancer cells through necrosis and pyroptosis as well as a transition in the TME from anti-inflammatory to pro-inflammatory, which stimulates a systemic anti-tumor immune response [125].
With the development of nanotechnology, there are promising application prospects for the use of nanocarriers loaded with antitumor drugs for the targeted therapy of breast cancer. Zhao et al. developed a biomimetic nanoparticle (BNP) loaded with indocyanine green and DAC. On the one hand, low-dose laser irradiation at the tumor site enhanced intracellular Ca2+ concentration, which triggers the release of cytochrome c and the activation of caspase-3 in breast cancer cells. On the other hand, the released DAC upregulates GSDME expression via suppressing DNA methylation, leading to powerful cancer cell pyroptosis [96]. Therefore, the nanoparticle is a very promising technique to show site-specific delivery of multiple drugs to result in combination therapeutic effects in breast cancer treatment [126].
Meanwhile, researchers have uncovered that several small-molecule chemicals or medications can induce pyroptosis in breast cancer cells and we summarize these pyroptosis-inducing drugs in Table 2.
Conclusions and future perspectives
Pyroptosis, a newly raised category of programmed cell death that is dependent on Gasdermin family proteins, has made further progress in recent years. Notably, a comprehensive understanding of the expression patterns and activities of the Gasdermin family members will assist in identifying the likelihood of pyroptosis in breast cancer cells and provide a foundation for future clinical translational research. However, in addition to being expressed in breast cancer cells, the Gasdermin family members are also abundantly expressed in normal human tissues [32, 127]. As a result, pyroptosis-inducing drugs will inevitably cause harm to normal tissue cells and a variety of undesirable effects when used on the human body. Therefore, when employing pyroptosis in the therapy of breast cancer, how to achieve a precise and effective treatment mode will be the focus of our follow-up research.
As described above, several traditional chemotherapeutic drugs (such as cisplatin, doxorubicin, etc.) have also been shown to induce pyroptosis in breast cancer cells, which not only kills tumor cells directly but also modifies the tumor microenvironment. This procedure assists in transforming “cold” cancers that are resistant to immunotherapy into “hot” ones that are responsive to immunotherapy. Besides, promising results have been shown when combining the function of pyroptosis with ICIs in breast cancer. For this reason, pyroptosis may offer innovative solutions for breast cancer immunotherapy.
Nevertheless, compared with the issues that have been resolved, additional mysteries remain to be addressed. Does the occurrence of pyroptosis increase or decrease the risk of breast cancer? How to weigh and avoid the adverse effects of pyroptosis inducers on breast cancer patients as much as possible? Is the effectiveness of pyroptosis inducers combined with ICIs limited to TNBC, or does it hold true for other molecular subtypes of breast cancer as well? Like the combination of pyroptosis with chemotherapy, is there a possibility of achieving a synergistic impact by combining endocrine therapy and anti-HER2 therapy with pyroptosis in the treatment of breast cancer? How does pyroptosis interact with other forms of PCD (autophagy, ferroptosis, etc.), and would combining pyroptosis with these other forms of PCD increase the therapeutic efficacy of breast cancer treatment? Exist any additional medications that efficiently induce pyroptosis in breast cancer cells and will be applied clinically? Many concerns must be solved before pyroptosis was formally applied in clinical settings.
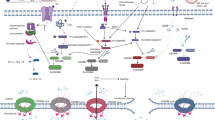
Taken together, our review summarizes the expression patterns and functions of Gasdermin family proteins in breast cancer and discussed the potential influence of pyroptosis on breast cancer progression and treatment strategies (Fig. 2). With a deeper knowledge of the physiological process and molecular mechanism of pyroptosis, more and more findings will be presented in the future. These will provide new ideas and methods for the choice of treatment decisions for breast cancer patients.
Chemotherapy, radiotherapy, and several small molecule chemicals or medications can induce pyroptosis in breast cancer cells, which results in cell lytic death, regulates cell proliferation and metastasis, and activates signaling pathways. Moreover, pyroptosis in breast cancer cells also exerts a crucial role in TME redistribution and is expected to improve the efficacy of ICIs.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300.
Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet 2021;397:1750–69.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Janku F, Mcconkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–39.
Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992;358:167–9.
Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–8.
Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–7.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660–5.
Kayagaki N, Stowe IB, Lee BL, O’rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666–71.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Shao F. Gasdermins: making pores for pyroptosis. Nat Rev Immunol. 2021;21:620–1.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Christgen S, Place DE, Kanneganti TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30:315–27.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016;535:153–8.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98.
Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity 2019;50:1352–64.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011;479:117–21.
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014;514:187–92.
Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–7.
Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–36.
Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 2015;43:923–32.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017;547:99–103.
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264–75.
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA. 2018;115:E10888–E10897.
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020;368:eaaz7548.
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020;579:415–20.
Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng Z, et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 2022;602:496–502.
Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20:384–405.
De Schutter E, Roelandt R, Riquet FB, Van Camp G, Wullaert A, Vandenabeele P. Punching holes in cellular membranes: biology and evolution of gasdermins. Trends Cell Biol. 2021;31:500–13.
Sarrio D, Martinez-Val J, Molina-Crespo A, Sanchez L, Moreno-Bueno G. The multifaceted roles of gasdermins in cancer biology and oncologic therapies. Biochim Biophys Acta Rev Cancer. 2021;1876:188635.
Wang M, Chen X, Zhang Y. Biological functions of gasdermins in cancer: from molecular mechanisms to therapeutic potential. Front Cell Dev Biol. 2021;9:638710.
Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11:718–24.
Moussette S, Al Tuwaijri A, Kohan-Ghadr HR, Elzein S, Farias R, Berube J, et al. Role of DNA methylation in expression control of the IKZF3-GSDMA region in human epithelial cells. PLoS ONE. 2017;12:e0172707.
Das S, Miller M, Broide DH. Chromosome 17q21 genes ORMDL3 and GSDMB in asthma and immune diseases. Adv Immunol. 2017;135:1–52.
Hergueta-Redondo M, Sarrio D, Molina-Crespo A, Megias D, Mota A, Rojo-Sebastian A, et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE. 2014;9:e90099.
Hergueta-Redondo M, Sarrio D, Molina-Crespo A, Vicario R, Bernado-Morales C, Martinez L, et al. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget 2016;7:56295–308.
Molina-Crespo A, Cadete A, Sarrio D, Gamez-Chiachio M, Martinez L, Chao K, et al. Intracellular delivery of an antibody targeting gasdermin-B reduces HER2 breast cancer aggressiveness. Clin Cancer Res. 2019;25:4846–58.
Watabe K, Ito A, Asada H, Endo Y, Kobayashi T, Nakamoto K, et al. Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells. Jpn J Cancer Res. 2001;92:140–51.
Wei J, Xu Z, Chen X, Wang X, Zeng S, Qian L, et al. Overexpression of GSDMC is a prognostic factor for predicting a poor outcome in lung adenocarcinoma. Mol Med Rep. 2020;21:360–70.
Cui YQ, Meng F, Zhan WL, Dai ZT, Liao X. High expression of GSDMC is associated with poor survival in kidney clear cell cancer. Biomed Res Int. 2021;2021:5282894.
Saeki N, Usui T, Aoyagi K, Kim DH, Sato M, Mabuchi T, et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48:261–71.
Xu D, Ji Z, Qiang L. Molecular characteristics, clinical implication, and cancer immunity interactions of pyroptosis-related genes in breast cancer. Front Med (Lausanne). 2021;8:702638.
Yuan S, Wang Y, Li Z, Chen X, Song P, Chen A, et al. Gasdermin D is involved in switching from apoptosis to pyroptosis in TLR4-mediated renal tubular epithelial cells injury in diabetic kidney disease. Arch Biochem Biophys. 2022;727:109347.
Bittner ZA, Schrader M, George SE, Amann R. Pyroptosis and its role in SARS-CoV-2 infection. Cells 2022;11:1717.
Lv T, Xiong X, Yan W, Liu M, Xu H, He Q. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J Immunother Cancer. 2022;10:e004763.
Berkel C, Cacan E. Differential expression and copy number variation of gasdermin (GSDM) family members, pore-forming proteins in pyroptosis, in normal and malignant serous ovarian tissue. Inflammation 2021;44:2203–16.
Wu X, Mao X, Huang Y, Zhu Q, Guan J, Wu L. Detection of proteins associated with the pyroptosis signaling pathway in breast cancer tissues and their significance. Int J Clin Exp Pathol. 2020;13:1408–14.
Yan H, Luo B, Wu X, Guan F, Yu X, Zhao L, et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. Int J Biol Sci. 2021;17:2606–21.
Ibrahim J, Op De Beeck K, Fransen E, Croes L, Beyens M, Suls A, et al. Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Med. 2019;8:2133–45.
Yokomizo K, Harada Y, Kijima K, Shinmura K, Sakata M, Sakuraba K, et al. Methylation of the DFNA5 gene is frequently detected in colorectal cancer. Anticancer Res. 2012;32:1319–22.
Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2007;98:88–95.
Xia Y, Jin Y, Cui D, Wu X, Song C, Jin W, et al. Antitumor effect of simvastatin in combination with DNA methyltransferase inhibitor on gastric cancer via GSDME-mediated pyroptosis. Front Pharm. 2022;13:860546.
Kim MS, Lebron C, Nagpal JK, Chae YK, Chang X, Huang Y, et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem Biophys Res Commun. 2008;370:38–43.
Fujikane T, Nishikawa N, Toyota M, Suzuki H, Nojima M, Maruyama R, et al. Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Res Treat. 2010;122:699–710.
Thompson DA, Weigel RJ. Characterization of a gene that is inversely correlated with estrogen receptor expression (ICERE-1) in breast carcinomas. Eur J Biochem. 1998;252:169–77.
Croes L, Beyens M, Fransen E, Ibrahim J, Vanden Berghe W, Suls A, et al. Large-scale analysis of DFNA5 methylation reveals its potential as biomarker for breast cancer. Clin Epigenet. 2018;10:51.
Croes L, De Beeck KO, Pauwels P, Vanden Berghe W, Peeters M, Fransen E, et al. DFNA5 promoter methylation a marker for breast tumorigenesis. Oncotarget 2017;8:31948–58.
Wang Y, Peng J, Mi X, Yang M. p53-GSDME elevation: a path for CDK7 inhibition to suppress breast cancer cell survival. Front Mol Biosci. 2021;8:697457.
Liao M, Qin R, Huang W, Zhu HP, Peng F, Han B, et al. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15:44.
Liu M, Wang L, Xia X, Wu Y, Zhu C, Duan M, et al. Regulated lytic cell death in breast cancer. Cell Biol Int. 2022;46:12–33.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
Tyutyunyk-Massey L, Gewirtz DA. Roles of autophagy in breast cancer treatment: target, bystander or benefactor. Semin Cancer Biol. 2020;66:155–62.
Seyrek K, Wohlfromm F, Espe J, Lavrik IN. The cross-talk of autophagy and apoptosis in breast carcinoma: implications for novel therapies? Biochem J. 2022;479:1581–608.
Sui S, Xu S, Pang D. Emerging role of ferroptosis in breast cancer: new dawn for overcoming tumor progression. Pharm Ther. 2022;232:107992.
An H, Heo JS, Kim P, Lian Z, Lee S, Park J, et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021;12:159.
Xia J, Chu C, Li W, Chen H, Xie W, Cheng R, et al. Mitochondrial protein UCP1 inhibits the malignant behaviors of triple-negative breast cancer through activation of mitophagy and pyroptosis. Int J Biol Sci. 2022;18:2949–61.
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997;80:421–34.
Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78:5243–58.
Jang JH, Kim DH, Lim JM, Lee JW, Jeong SJ, Kim KP, et al. Breast cancer Cell-derived soluble CD44 promotes tumor progression by triggering macrophage IL1beta production. Cancer Res. 2020;80:1342–56.
Pham DV, Raut PK, Pandit M, Chang JH, Katila N, Choi DY, et al. Globular adiponectin inhibits breast cancer cell growth through modulation of inflammasome activation: critical role of Sestrin2 and AMPK signaling. Cancers (Basel). 2020;12:613.
Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016;7:75571–84.
Tulotta C, Lefley DV, Freeman K, Gregory WM, Hanby AM, Heath PR, et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin Cancer Res. 2019;25:2769–82.
Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10:4375.
Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee HC, et al. Zerumbone suppresses IL-1beta-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytother Res. 2014;28:1654–60.
Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis. 2015;32:335–44.
Perez-Yepez EA, Ayala-Sumuano JT, Lezama R, Meza I. A novel beta-catenin signaling pathway activated by IL-1beta leads to the onset of epithelial–mesenchymal transition in breast cancer cells. Cancer Lett. 2014;354:164–71.
Wang Y, Zhang H, Xu Y, Peng T, Meng X, Zou F. NLRP3 induces the autocrine secretion of IL-1beta to promote epithelial–mesenchymal transition and metastasis in breast cancer. Biochem Biophys Res Commun. 2021;560:72–9.
Storr SJ, Safuan S, Ahmad N, El-Refaee M, Jackson AM, Martin SG. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunol Immunother. 2017;66:1287–94.
You D, Jeong Y, Yoon SY, A Kim S, Kim SW, Nam SJ, et al. Celastrol attenuates the inflammatory response by inhibiting IL1beta expression in triplenegative breast cancer cells. Oncol Rep. 2021;45:89.
Eyre R, Alferez DG, Santiago-Gomez A, Spence K, Mcconnell JC, Hart C, et al. Microenvironmental IL1beta promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat Commun. 2019;10:5016.
Nisar MA, Zheng Q, Saleem MZ, Ahmmed B, Ramzan MN, Ud Din SR, et al. IL-1beta promotes vasculogenic mimicry of breast cancer cells through p38/MAPK and PI3K/Akt signaling pathways. Front Oncol. 2021;11:618839.
Voloshin T, Alishekevitz D, Kaneti L, Miller V, Isakov E, Kaplanov I, et al. Blocking IL1beta pathway following paclitaxel chemotherapy slightly inhibits primary tumor growth but promotes spontaneous metastasis. Mol Cancer Ther. 2015;14:1385–94.
Castano Z, San Juan BP, Spiegel A, Pant A, Decristo MJ, Laszewski T, et al. IL-1beta inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat Cell Biol. 2018;20:1084–97.
El-Deeb MMK, El-Sheredy HG, Mohammed AF. The possible role of Interleukin (IL)-18 and nitrous oxide and their relation to oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev. 2019;20:2659–65.
Gunel N, Coskun U, Sancak B, Hasdemir O, Sare M, Bayram O, et al. Prognostic value of serum IL-18 and nitric oxide activity in breast cancer patients at operable stage. Am J Clin Oncol. 2003;26:416–21.
Li K, Wei L, Huang Y, Wu Y, Su M, Pang X, et al. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol. 2016;48:2479–87.
Yang Y, Cheon S, Jung MK, Song SB, Kim D, Kim HJ, et al. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun. 2015;459:379–86.
Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Disco. 2021;11:933–59.
Yang Q, Ma X, Xiao Y, Zhang T, Yang L, Yang S, et al. Engineering prodrug nanomicelles as pyroptosis inducer for codelivery of PI3K/mTOR and CDK inhibitors to enhance antitumor immunity. Acta Pharm Sin B 2022;12:3139–55.
Niu X, Chen L, Li Y, Hu Z, He F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: perspectives for immunotherapy of SCLC. Semin Cancer Biol. 2022;86:273–85.
Jin J, Yuan P, Yu W, Lin J, Xu A, Xu X, et al. Mitochondria-targeting polymer micelle of dichloroacetate induced pyroptosis to enhance osteosarcoma immunotherapy. ACS Nano. 2022;16:10327–40.
Zhao P, Wang M, Chen M, Chen Z, Peng X, Zhou F, et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 2020;254:120142.
Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 2020;579:421–6.
Gelfo V, Romaniello D, Mazzeschi M, Sgarzi M, Grilli G, Morselli A, et al. Roles of IL-1 in cancer: from tumor progression to resistance to targeted therapies. Int J Mol Sci. 2020;21:6009.
Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90.
Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, et al. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci USA. 2019;116:1361–9.
Park IH, Yang HN, Lee KJ, Kim TS, Lee ES, Jung SY, et al. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget 2017;8:32722–30.
Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–60.
Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006;26:174–9.
Hernandez AP, Juanes-Velasco P, Landeira-Vinuela A, Bareke H, Montalvillo E, Gongora R, et al. Restoring the immunity in the tumor microenvironment: insights into immunogenic cell death in onco-therapies. Cancers (Basel). 2021;13:2821.
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8.
Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6:1962–5.
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44.
Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25:8159–68.
Fan JX, Deng RH, Wang H, Liu XH, Wang XN, Qin R, et al. Epigenetics-based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. Nano Lett. 2019;19:8049–58.
Li L, Tian H, Zhang Z, Ding N, He K, Lu S, et al. Carrier-free nanoplatform via evoking pyroptosis and immune response against breast cancer. ACS Appl Mater Interfaces. 2023;15:452–68.
O’meara TA, Tolaney SM. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 2021;12:394–400.
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70.
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–7.
Tian T, Han J, Huang J, Li S, Pang H. Hypoxia-induced intracellular and extracellular heat shock protein gp96 increases paclitaxel-resistance and facilitates immune evasion in breast cancer. Front Oncol. 2021;11:784777.
Su W, Qiu W, Li SJ, Wang S, Xie J, Yang QC, et al. Dual-responsive STAT3 inhibitor nanoprodrug combined with oncolytic virus elicits synergistic antitumor immune responses by igniting pyroptosis. Adv Mater. 2022:e2209379. https://doi.org/10.1002/adma.202209379.
Elion DL, Jacobson ME, Hicks DJ, Rahman B, Sanchez V, Gonzales-Ericsson PI, et al. Therapeutically active RIG-I agonist induces immunogenic tumor cell killing in breast cancers. Cancer Res. 2018;78:6183–95.
Winer EP, Lipatov O, Im SA, Goncalves A, Munoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511.
Salas-Benito D, Perez-Gracia JL, Ponz-Sarvise M, Rodriguez-Ruiz ME, Martinez-Forero I, Castanon E, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11:1353–67.
Voorwerk L, Slagter M, Horlings HM, Sikorska K, Van De Vijver KK, De Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–8.
Schmid P, Cortes J, Pusztai L, Mcarthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090–100.
Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33:534–43.
Jimenez-Garduno AM, Mendoza-Rodriguez MG, Urrutia-Cabrera D, Dominguez-Robles MC, Perez-Yepez EA, Ayala-Sumuano JT, et al. IL-1beta induced methylation of the estrogen receptor ERalpha gene correlates with EMT and chemoresistance in breast cancer cells. Biochem Biophys Res Commun. 2017;490:780–5.
Cao W, Chen G, Wu L, Yu KN, Sun M, Yang M, et al. Ionizing radiation triggers the antitumor immunity by inducing gasdermin E-mediated pyroptosis in tumor cells. Int J Radiat Oncol Biol Phys. 2023;115:440–52.
Ringel-Scaia VM, Beitel-White N, Lorenzo MF, Brock RM, Huie KE, Coutermarsh-Ott S, et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine 2019;44:112–25.
Liu Y, Guo K, Ding M, Zhang B, Xiao N, Tang Z, et al. Engineered magnetic polymer nanoparticles can ameliorate breast cancer treatment inducing pyroptosis-starvation along with chemotherapy. ACS Appl Mater Interfaces. 2022;14:42541–57.
Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57.
Sun K, Chen RX, Li JZ, Luo ZX. LINC00511/hsa-miR-573 axis-mediated high expression of Gasdermin C associates with dismal prognosis and tumor immune infiltration of breast cancer. Sci Rep. 2022;12:14788.
Pizato N, Luzete BC, Kiffer L, Correa LH, De Oliveira Santos I, Assumpcao JAF, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952.
Jiao Y, Wang L, Lu L, Liu J, Li X, Zhao H, et al. The role of caspase-4 and NLRP1 in MCF7 cell pyroptosis induced by hUCMSC-secreted factors. Stem Cells Int. 2020;2020:8867115.
Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-kappaB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19:1089–104.
Wang JG, Jian WJ, Li Y, Zhang J. Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. Kaohsiung J Med Sci. 2021;37:572–82.
Yan L, Liu Y, Ma XF, Hou D, Zhang YH, Sun Y, et al. Triclabendazole induces pyroptosis by activating caspase-3 to cleave GSDME in breast cancer cells. Front Pharm. 2021;12:670081.
Tang J, Bei M, Zhu J, Xu G, Chen D, Jin X, et al. Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ Toxicol Pharm. 2021;87:103686.
Li Y, Wang W, Li A, Huang W, Chen S, Han F, et al. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem Biol Interact. 2021;340:109434.
Xu W, Song C, Wang X, Li Y, Bai X, Liang X, et al. Downregulation of miR-155-5p enhances the anti-tumor effect of cetuximab on triple-negative breast cancer cells via inducing cell apoptosis and pyroptosis. Aging (Albany, NY). 2021;13:228–40.
Tamura Y, Morikawa M, Tanabe R, Miyazono K, Koinuma D. Anti-pyroptotic function of TGF-beta is suppressed by a synthetic dsRNA analogue in triple negative breast cancer cells. Mol Oncol. 2021;15:1289–307.
Zhang F, Liu Q, Ganesan K, Kewu Z, Shen J, Gang F, et al. The antitriple negative breast cancer efficacy of Spatholobus suberectus Dunn on ROS-induced noncanonical inflammasome pyroptotic pathway. Oxid Med Cell Longev. 2021;2021:5187569.
Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma D, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34:581–94.e588.
Ma L, Bian M, Gao H, Zhou Z, Yi W. A novel 3-acyl isoquinolin-1(2H)-one induces G2 phase arrest, apoptosis and GSDME-dependent pyroptosis in breast cancer. PLoS ONE. 2022;17:e0268060.
Xu T, Wang Z, Liu J, Wang G, Zhou D, Du Y, et al. Cyclin-dependent kinase inhibitors function as potential immune regulators via inducing pyroptosis in triple negative breast cancer. Front Oncol. 2022;12:820696.
Li Y, Wu J, Qiu X, Dong S, He J, Liu J, et al. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact Mater. 2023;20:548–60.
Zhu Y, Yue P, Dickinson CF, Yang JK, Datanagan K, Zhai N, et al. Natural product preferentially targets redox and metabolic adaptations and aberrantly active STAT3 to inhibit breast tumor growth in vivo. Cell Death Dis. 2022;13:1022.
Chang M, Wang Z, Dong C, Zhou R, Chen L, Huang H, et al. Ultrasound-amplified enzyodynamic tumor therapy by perovskite nanoenzyme-enabled cell pyroptosis and cascade catalysis. Adv Mater. 2022:e2208817. https://doi.org/10.1002/adma.202208817.
Acknowledgements
Figures were created using BioRender.com. Retrieved from https://app.biorender.com.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ20H160018), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant No. 2022RC222), and the Traditional Chinese Medical Science and Technology Plan of Zhejiang Province (Grant No. 2020ZQ036).
Author information
Authors and Affiliations
Contributions
Manuscript writing (original draft): CC and QY. Conceptualization/funding acquisition: JL, JG, and SH. Manuscript review and editing: LW and JZ. Manuscript revision: AX and XL. Tables and figures editing: TR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors agreed to publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, C., Ye, Q., Wang, L. et al. Targeting pyroptosis in breast cancer: biological functions and therapeutic potentials on It. Cell Death Discov. 9, 75 (2023). https://doi.org/10.1038/s41420-023-01370-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01370-9