Abstract
Mesenchymal stem cells (MSCs) are widely distributed pluripotent stem cells with powerful immunomodulatory capacity. MSCs transplantation therapy (MSCT) is widely used in the fields of tissue regeneration and repair, and treatment of inflammatory diseases. Apoptosis is an important way for tissues to maintain cell renewal, but it also plays an important role in various diseases. And many studies have shown that MSCs improves the diseases by regulating cell apoptosis. The regulation of MSCs on apoptosis is double-sided. On the one hand, MSCs significantly inhibit the apoptosis of diseased cells. On the other hand, MSCs also promote the apoptosis of tumor cells and excessive immune cells. Furthermore, MSCs regulate apoptosis through multiple molecules and pathways, including three classical apoptotic signaling pathways and other pathways. In this review, we summarize the current evidence on the regulation of apoptosis by MSCs.
Similar content being viewed by others
Facts
-
MSCs protect tissue cells from apoptosis and improve diseases through apoptosis regulation pathways.
-
MSCs promote specific cell apoptosis to combat autoimmune diseases and tumors.
-
The apoptosis of MSCs themselves or surrounding tissue cells also helps enhance the therapeutic effect.
Open Questions
-
What are the most key molecules for the antiapoptotic effect of MSCs, and how to stabilize and enhance the antiapoptotic effect of MSCs?
-
What are the effects of MSCs on other forms of programmed cell death?
-
How to explore the intrinsic mechanisms and potential clinical application strategies of apoptosis in MSCs?
-
How to enhance the therapeutic effects of MSCs through apoptotic products?
Introduction
Mesenchymal stem cells (MSCs) transplantation therapy (MSCT) has been widely recognized as an effective clinical strategy for a variety of diseases [1]. And the therapeutic effect of MSCs mainly depends on several factors and molecules, including immunomodulatory molecules, chemokines, growth factors, and non-coding RNAs (ncRNAs) [2,3,4]. The factors and molecules affect the proliferation, apoptosis, immune homeostasis and metabolic balance of disease-related cells. Through the mediators, MSCs reduce disease damage and improve regeneration potential by inhibiting abnormal apoptosis and promoting tissue cell proliferation. In addition, MSCs have a strong immunoregulatory capacity, which effectively regulate the immune homeostasis of disease tissues and protect normal tissue cells from damage [1, 5]. However, there remains a lack of understanding of the above processes, which hampers the further clinical application of MSCs.
Apoptosis is a complex programmed cellular death process that involves many molecules and pathways [6]. Physiological apoptosis maintains homeostasis by removing senescent cells and abnormal cells, but pathological apoptosis is an important factor in the occurrence and development of diseases [7]. In ischemic-reperfusion injuries, hemorrhagic diseases, and neurodegenerative diseases, excessive apoptosis of cells is a key factor in the development of the diseases [8,9,10]. In addition, resistance of tumor cells to apoptosis is an important contributor to tumor proliferation and invasion [11, 12]. Therefore, correcting the abnormal apoptosis process of cells is an important method to alleviate the diseases.
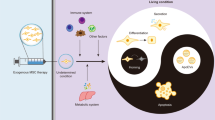
MSCs regulate the apoptosis of the cells through multiple molecules and pathways (Fig. 1). Many studies have found that MSCT significantly reduce the apoptosis of diseases-related tissue cells (Table 1), which is achieved by the activation of a variety of signaling pathways [13,14,15]. More interestingly, MSCs also alleviate diseases by promoting apoptosis in some cells, such as autoimmune diseases and tumors [16,17,18]. These evidences suggest that MSCs exert therapeutic effects by regulating the apoptotic process of various cells. In this review, we summarize the current understanding of the role of MSCs in the regulation of apoptosis, and provide a new perspective on the relationship between MSCs and apoptosis.
The influence of MSCs on apoptosis varies depending on the target cells. MSCs typically demonstrate significant antiapoptotic effects on cells that have been damaged by disease or trauma. However, MSCs can also promote the apoptosis of immune cells and tumor cells. These findings highlight the crucial role of MSCs in the intricate regulatory network of apoptosis.
MSCs protect cells from apoptosis
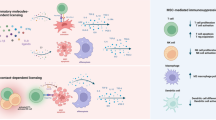
Inhibition of apoptosis is an important mechanism of MSCs to alleviate various diseases, including cardiovascular diseases, renal injury, neurodegenerative diseases, and premature ovarian failure [19,20,21,22]. In these diseases, the number of normal cells undergoing pathological apoptosis is closely related to the severity of the disease. Moreover, many studies have found that the apoptosis of these cells mainly through three pathways, including endogenous pathway, exogenous pathway, and endoplasmic reticulum pathway [23, 24]. And MSCs regulate the above three apoptotic pathways through various mechanisms to significantly reverse apoptotic events in various pathological states (Fig. 2).
MSCs of various origins influence the outcome of apoptosis by targeting multiple apoptosis regulatory pathways. This is mainly achieved through their secreted components, which include various ncRNAs and cytokines. These antiapoptotic mediators significantly inhibit caspase cascade in target cells through endogenous pathways, exogenous pathways, and endoplasmic reticulum stress pathways, thereby preventing apoptosis. Upstream signaling pathways of apoptosis regulation, such as the PI3K pathway and p53 pathway, are also among the targets of regulation. In addition, MSCs also exert antiapoptotic effects through the regulation of mitochondria and autophagy.
Endogenous pathway (mitochondrial pathway)
Endogenous pathway refers to apoptotic events induced by changes in mitochondrial membrane permeability, also known as mitochondrial pathway. The increased permeability of the mitochondrial membrane leads to the release of pro-apoptotic factors in the mitochondria into the cytoplasm, which activates the caspase cascade and initiates the apoptotic process [25, 26]. And b-cell lymphoma-2 (Bcl-2) family proteins are the main regulators of mitochondrial pathway, which regulate the permeability of mitochondrial membrane [27]. The Bcl-2 family includes antiapoptotic proteins Bcl-2 and Bcl-xl, pro-apoptotic proteins Bad, Bid, Bax, and Bim. And multiple factors derived from MSCs control the permeability of mitochondrial membrane and thus regulate apoptosis by regulating the differential expression of Bcl-2 family.
Bcl-2 and Bax are the most reported apoptotic indicator proteins. Many studies have found that multiple mediators derived from MSCs significantly increase antiapoptotic protein Bcl-2 and decrease pro-apoptotic protein Bax in disease cells, including lnterleukin-6 (IL-6), prostaglandin E2, transforming growth factor-beta, microRNA (miR)-29a-3p, and miR-125b-5p [28,29,30,31,32]. And miR-93, miR-150-5p, and long ncRNA-UCA1 from MSCs also promote the expression and recovery of Bcl-2 in target cells [33,34,35]. In addition, the conditioned medium of MSCs enhance translocation of Bcl-2 to the nucleus in mouse alveolar epithelial cells [36]. The findings suggest that MSCs have strong regulatory capabilities on both Bcl-2 and Bax. Moreover, MSCs also inhibit apoptosis by regulating the expression of Bcl-xl and Bad [37,38,39]. Therefore, the Bcl-2 family is an important center for the regulation of apoptosis by MSCs in the mitochondrial pathway.
Exogenous pathway (Death receptor pathway)
Exogenous pathway refers to death receptor-mediated apoptotic events, also known as death receptor pathway. There are five major death receptors, including Fas, tumor necrosis factor receptor, death receptor (DR) 3, DR4, and DR5, and their corresponding ligands include Fas-L, tumor necrosis factor (TNF), DR3L, and TNF-related apoptosis inducing ligand (TRAIL). Specifically, the extracellular apoptotic signals activate the intracellular caspase cascade by activating different death receptors. And these death receptors and related signaling systems are also important targets for MSCs to exert antiapoptotic effects. For example, MSCT significantly reduce the TNF-α, inducible nitric oxide synthase, Fas-L, and other pro-apoptotic signals in the cell microenvironment, and finally alleviate the apoptosis of cells [40, 41]. Moreover, the miR-17 derived from MSCs-extracellular vesicles (EVs) regulates bromodomain-containing protein 4-mediated enhancer of zeste homolog 2 (EZH2)/TRAIL axis to essentially inhibit lipopolysaccharide (LPS)-induced inflammation and apoptosis of RAW264.7 cells [42]. Similarly, MSCs effectively inhibit alveolar macrophage apoptosis and reverse LPS-induced lung injury by reducing toll-like receptor (TLR) 3 mediated mitogen-activated protein kinase (MAPK) and NF-κB signaling [43]. These evidences suggest that MSCs inhibit death receptor-mediated apoptosis by reducing apoptotic signals and regulating death receptor apoptotic signaling pathway.
Endoplasmic reticulum pathway
Endoplasmic reticulum stress (ERS) refers to an increase in misfolded proteins resulting from impaired endoplasmic reticulum function, and long-term ERS induce apoptosis [24]. Studies have found that hepatocyte growth factor (HGF) and TNF-inducible gene 6 protein secreted by MSCs significantly inhibit ERS and its subsequent pro-apoptotic and pro-inflammatory consequences [44, 45]. Moreover, miR-21 derived from MSCs-exosomes (MSCs-Exos) effectively inhibit hypoxia-induced apoptosis by alleviating ERS and inhibiting phosphorylation of p38 MAPK [46]. MSCs protect the islets after transplantation from ERS-induced apoptosis and improved the viability of the islets [47]. These findings reveal that MSCs can inhibit ERS and thus alleviate apoptosis.
Upstream regulatory pathways
In addition to the three major apoptotic signaling pathways mentioned above, there are other pathways that control apoptosis by regulating the expression of survival and apoptosis-related genes. According to regulated genes, these pathways should be divided into two categories: antiapoptotic pathway and pro-apoptotic pathway. And these pathways are also important targets for MSCs to exert antiapoptotic effects.
Antiapoptotic pathway
Phosphatidylinositol 3-kinase (PI3K)-AKT is an important pathway for cell survival, and its activation upregulate the expression of many antiapoptotic genes and proliferative genes [48]. Moreover, cancer suppressor gene PTEN dephosphorylates AKT and reduces its activation, which is a negative regulator of PI3K/AKT pathway. However, MSCs-derived miR-29b-3p, miR-223, miR-144, and miR-486-5p activate PI3K/AKT pathway by inhibiting PTEN, thus inhibiting apoptosis [49,50,51,52]. Similarly, long ncRNA KLF-AS1 and miR-132-3p derived from MSCs-Exos also activate the PI3K/AKT pathway and exert antiapoptotic effects [53, 54]. These findings show that MSCs protect cells from apoptosis by secreting various factors to activate the PI3K/AKT signaling pathway.
Pro-apoptotic pathway
In the apoptotic signaling network, the p53 signaling pathway regulates the expression of pro-apoptotic genes, including Bax, Bak, Bad, and Apaf-1. However, miR-369-3p, miR-644-5p, and miR-125b-5p in MSCs-Exos exert antiapoptotic effects by inhibiting the activation of p53 [32, 55,56,57]. And interestingly, miR-455-3p and miR-19a exhibit antiapoptotic effects by inhibiting JNK and subsequent activation of p53 and caspase-3 [58, 59]. Additionally, MSCs significantly alleviate cisplatin-induced toxicity and improve islet viability by inhibiting p38/MAPK pathway [46, 60,61,62]. These findings indicate that MSCs synergistically exert an inhibitory effect on apoptosis by inhibiting the pro-apoptotic pathway while enhancing the antiapoptotic pathway.
Others
MSCs also resist apoptosis in some interesting ways, including by regulating mitochondria and autophagy. Li, X et al. and Li, H et al. have found that MSCs protect airway smooth muscle cells and injured neurons from apoptosis by mitochondrial transfer [63, 64]. And MSCs also show the ability to regulate mitochondrial potential, reduce mitochondrial stress, and reduce mitochondrial damage to inhibit apoptosis [65, 66]. In addition, promoting mitochondrial protective autophagy is also an important way that MSCs resist apoptosis [67, 68]. Notably, the protective autophagy induced by MSCs is not only in the mitochondria but also in the whole cell. MSCs enhance autophagy flux by inhibiting mTOR signal activation, which promote autophagy and inhibit apoptosis [69, 70]. And forkhead box O3 and ALKBH5 are also targets of MSCs mediated protective autophagy to inhibit apoptosis [71, 72]. Therefore, MSCs fight apoptosis by regulating mitochondrial biological function and promoting protective autophagy.
Resistance to NOD-like receptor thermal protein domain associated protein 3 (NLRP3)-mediated pyroptosis is an interesting extension of the ability of MSCs to inhibit apoptosis. MSCT significantly decrease inflammasome-related pyroptosis markers including cleaved caspase-1, gasdermin D, NLRP3, IL-1beta, and IL-18 in diseases [73,74,75]. And MSCs-Exos also play a key role in this process. MSCs-Exos upregulate the expression of FOXO3a, which inhibit pyroptosis and the release of inflammatory cytokines [74, 76]. In MSCs-Exos, ciric-003564, miR-539-5p, ciric-HIPK3, miR-223-3p, and miR-26a-5p are all involved in the inhibition of NLRP3-mediated pyroptosis by MSCs [75,76,77,78,79]. These ncRNAs greatly enhance the remission effect of MSCs on various pyroptosis diseases, and suggest that MSCs have great potential to combat inflammatory apoptosis.
MSCs improve diseases by inhibiting apoptosis
Inhibition of tissue cell apoptosis is a key mechanism in the therapeutic effect of MSCs, which enables MSCT to improve many diseases. These diseases involve the following tissues and organs, including heart, liver, lung, and nervous system. And Ischemia-reperfusion injury (IRI) is a common factor inducing abnormal apoptosis in these tissues and organs [80]. Interestingly, MSCs can significantly improve the tissue and organ dysfunction caused by these diseases through inhibiting abnormal apoptosis and inflammatory responses.
Heart diseases
Myocardial infarction (MI) is a common heart disease in which myocardial cells undergo IRI, which often induces abnormal apoptosis of cardiomyocytes [81]. MSCs-derived medium and exosomes are considered as new biological drug for the treatment of MI. And various ncRNAs in the culture medium and exosomes have significant therapeutic effects by inhibiting cardiomyocyte apoptosis, including miR-150-3p, miR-144, miR-486-5p, miR-455-3p, miR-19a, miR-25-3p, miR-185, miR-221/222, and lncRNA-KLF3-AS1 [34, 51, 52, 58, 59, 82,83,84,85]. By secreting these ncRNAs, MSCT significantly inhibit myocardial cell apoptosis and fibrosis, reduce inflammation, and improve myocardial function. Notably, enhancing the cardioprotective effects of MSCs by genetic modification or gene editing also is a welcome approach [19]. In particular, various molecules with cardioprotective effects, include n-cadherin, lipocalin 2, c1q/tumor necrosis factor-related protein 3, follistatin-like 1, stromal-derived factor 1, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 4, and glucagon-like peptide-1 [86,87,88,89,90,91,92]. In addition, pretreatment of MSCs before transplantation in vitro also achieve similar enhancement effects by enhancing apoptosis resistance, such as hypoxia pretreatment, interferon (IFN)-γ pretreatment, atorvastatin pretreatment, sphingosine 1-phosphate pretreatment, and the combined pretreatment of HGF and insulin-like growth factor 1 [35, 93,94,95,96]. These findings suggest that enhancing the antiapoptotic ability of MSCs has a broad prospect in alleviating myocardial cell injury caused by MI.
Liver diseases
Liver transplantation is an effective strategy for the treatment of various end-stage liver diseases; however, IRI of hepatocytes often occurs during this process [97]. Emerging evidences suggest that MSCs have a strong hepatoprotective effect, which helps to alleviate hepatic IRI, liver failure, and liver fibrosis [98,99,100]. The hepatoprotective effect is mainly reflected in inhibiting hepatocyte apoptosis, promoting hepatocyte proliferation, inhibiting liver inflammation, and oxidative stress. Various secretory mediators derived from MSCs significantly inhibit liver injury, improve the success rate of liver transplantation, promote liver regeneration, and improve liver function by playing a hepatoprotective effect [28, 101,102,103]. These mediators include IL-6, prostaglandin E2, ransforming growth factor-beta, and ncRNAs [28,29,30, 104]. In addition, heme oxygenase 1 modification and hypoxic preconditioning significantly enhance the hepatoprotective effect of MSCs [105, 106]. Therefore, MSCT has a significant alleviating effect on hepatocyte apoptosis caused by acute liver injury.
Lung diseases
MSCT is also highly effective for various lung diseases through key mechanisms such as inhibition of apoptosis. Interestingly, the apoptotic protective effect of MSCs on alveolar epithelial cells has a broad range of disease applications, including acute respiratory distress syndrome or lung injury induced by IRI, smoke, influenza virus, sulfur mustard, and radiation [107,108,109,110,111,112]. In addition, MSCT also protect against pulmonary fibrosis induced by diabetes, silicosis, and bleomycin [113,114,115]. Moreover, the major protective mediators are mainly derived from the secretome of MSCs [36]. Therefore, MSCs have a wide range of effects to protect lung against various injury, including inhibiting alveolar epithelial cell apoptosis and fibrosis, inhibiting inflammation, reducing lung injury, and promoting the recovery of alveolar barrier function.
Neurological diseases
Hypoxic-ischemic encephalopathy and spinal cord injury are important causes of neuronal apoptosis, which can also be mitigated by MSCs. In the in vivo rat middle cerebral artery occlusion model and in vitro neuronal oxygen and glucose deprivation experiments, MSCs significantly inhibit abnormal apoptosis of neuronal and microglial, and improve neurobehavioral deficits [33, 116]. And MSCs-derived exosomes or vesicles are the key mediators in this process [21]. In addition to secreting vesicles, an interesting mechanism by which MSCs alleviate spinal cord injury induced neuronal apoptosis is by mitochondrial transfer [117]. Mitochondria derived MSCs transfer to damaged neurons not only inhibit apoptosis but also promote axon regeneration, which improve motor recovery. Moreover, MSCs also alleviate Alzheimer disease by reducing inflammation, inhibiting apoptosis, and regulating autophagy [118].
Other diseases
There are also diseases involving other organs or tissues that can be alleviated by MSCs through inhibiting apoptosis, including kidney injury, premature ovarian failure, pancreatitis, intervertebral disc degeneration, and osteoarthritis [67, 119,120,121,122]. Similar to the aforementioned diseases, MSCs significantly alleviate abnormal tissue cell apoptosis, reduce tissue inflammation and restore organ function in these diseases. In general, the antiapoptotic effect of MSCs has the following characteristics. The antiapoptotic ability of MSCs mainly alleviates two types of apoptosis-related diseases, instantaneous massive apoptosis due to acute injury and persistent apoptosis due to chronic injury. The remission of MSCs on these two apoptotic diseases mainly depends on blocking the apoptotic process and alleviating the inflammatory microenvironment. Moreover, the antiapoptotic mechanisms of MSCs are mainly mediated through their secretome, including ncRNAs, cytokines and mitochondria. MSCs secrete these mediators to inhibit apoptosis and reduce inflammation, which are the main therapeutic effects of MSCs on the disease. In addition, in vitro pretreatment or gene modification can enhance the effect of MSCT by enhancing the survival ability of MSCs or carrying protective components. These characteristics make MSCT have strong efficacy to be used in the treatment of many diseases.
MSCs enhance apoptosis of target cells
MSCs promote apoptosis of tumor cells
In addition to the aforementioned MSCs promote tumor growth by inhibiting tumor cell apoptosis, MSCs also are found to promote tumor cell apoptosis and inhibit tumor growth (Table 2). For example, miR-23b-5p derived from MSCs-Exos significantly reduce the proliferation and induce apoptosis of acute myeloid leukemia cells by reversing the TRIM14-activated PI3K/AKT pathway [123]. And miR-205 retards prostate cancer progression by inhibiting rhophilin Rho GTPase binding protein 2 [124]. Similarly, TNF-α-induced MSCs upregulate TRAIL expression and induce apoptosis in triple-negative breast cancer MDA-MB-231 (MDA) cells by secreting IFN-β [125]. And MSCs with highly expressed Fas-L significantly induce apoptosis in multiple myeloma cells [126]. Moreover, MSCs have also been shown to have significant pro-apoptotic effects on a variety of other tumor cells, including glioma U251 cells, pancreatic cancer cell, hepatocellular carcinoma cells, and lymphoma cells [127,128,129,130]. Therefore, MSCs have a strong pro-apoptotic effect on a variety of tumor cells through various pathways. These findings show the broad application prospect of MSCs in the field of tumor therapy; however, it also suggests that we need to further explore the relationship between MSCs and tumor cells.
MSCs promote apoptosis of immune cells and other cells
MSCs also alleviate various autoimmune diseases by promoting apoptosis of immune cells. The pro-apoptotic effect of MSCs on immune cells is usually regarded as part of the immunosuppressive ability of MSCs. And emerging evidences suggest that MSCs significantly inhibit T cells to exert immunosuppressive activity, which is involved in a variety of immune molecules, including indoleamine (2,3)-dioxygenase, programmed cell death 1 ligand 1, and Fas-L [18, 131,132,133]. These immune molecules help MSCs effectively alleviate graft-versus-host disease, systemic sclerosis, and DSS-induced ulcerative colitis. In addition, in vitro experiments also find that MSCs promote hepatic stellate cells apoptosis and help to alleviate the process of liver fibrosis [134, 135]. Many ncRNAs are also involved in the remission of autoimmune diseases. MSCs-derived exosomes suppress miR-5189-3p to facilitate the apoptosis of fibroblast-like synoviocytes via the basic leucine zipper ATF-like transcription factor 2/JAK2/STAT3 signaling pathway, which facilitate relieve ankylosing spondylitis (AS) [136]. The MSCs-secreted miR-26a inhibit the proliferation of high glucose-induced human skin fibroblasts cells and promote cell apoptosis, which may be related to the TLR4/NF-κB signaling pathway [137]. These above evidences indicate that the pro-apoptotic effect of MSCs on immune cells is also an integral part of the efficacy of MSCs.
Self-regulation of apoptosis by MSCs
The apoptosis of MSCs themselves is closely related to the therapeutic effects of MSCT and the treatment of various diseases. Regulation of MSCs apoptosis involves multiple molecular and signaling pathways (Table 3). Several factors, such as hypoxia/serum deprivation, hydrogen peroxide, dexamethasone, and metformin, can induce MSC apoptosis [138,139,140,141]. Nonetheless, there are various measures that can alleviate this apoptotic effect, including drug induction, exogenous factor pretreatment, overexpression of genes or ncRNAs, and more [142,143,144,145]. Therefore, The primary factors that pose a threat to MSC apoptosis are hypoxia, oxidative stress, and drug toxicity, while the rescue measures primarily target key signaling molecular pathways, including ERK, MAPK, NRF2, PI3K/AKT, and the Bcl-2 family.
Although MSCs undergo apoptosis due to various reasons, interestingly, apoptotic MSCs also possess significant biological functions and therapeutic potential. Researches have shown that the MSCs injected into the body during MSCT therapy undergo widespread apoptosis and induce receptor-mediated immune regulation within the body, which is closely related to the therapeutic effects of MSCT [146, 147]. Further researches highlight that apoptotic vesicles derived from MSCs (MSC-ApoVs) possess various functions and promising applications, including immune regulation, promotion of proliferation and tissue regeneration, homeostasis maintenance, and drug delivery[148, 149]. This is mainly based on the molecules transferred by MSC-ApoVs [147, 150], and the immune response after immune cell engulfment of MSC-ApoVs [151, 152]. These findings broaden the therapeutic strategies of MSCT and deepen our understanding of MSC-ApoVs.
Moreover, MSCs have demonstrated the ability to exert biological effects through the engulfment of apoptotic cells. This phenomenon occurs when apoptotic cells stimulate MSCs to target apoptotic sites via the HGF/c-Met axis [153, 154]. Furthermore, the presence of circulating apoptotic bodies contributes to the self-renewal and osteogenic differentiation of bone marrow MSCs by delivering cytokines [155]. Additionally, apoptotic cells induce MSCs to actively suppress T-cell immunity through the COX2/PGE2 axis [156]. Consequently, exploring the interaction between MSCs and apoptosis will provide valuable insights into the biological functions of MSCs and the therapeutic potential of MSCT.
Insights into the dual regulatory effects of MSCs on apoptosis
MSCs exhibit a dualistic characteristic in the regulation of apoptosis, displaying both inhibitory and promotive effects that can be attributed to various factors. Initially, depending on the specific cytokine stimulation, MSCs can exert diverse or even contradictory effects. For instance, interferon-gamma activates MSCs and promotes anti-inflammatory and antiapoptotic effects by inducing the release of various anti-inflammatory factors and growth factors that inhibit inflammatory responses and reduce cell death [157]. Conversely, certain cytokines such as LPS may stimulate MSCs to secrete signaling molecules that enhance inflammation and increase apoptosis [158]. In such cases, MSCs may contribute to promoting inflammatory responses and facilitating apoptotic processes.
Subsequently, MSCs are characterized by their heterogeneity and encompass various subsets that bestow them with remarkable adaptability. Sun et al. and Wang et al. identified 7 tissue-specific and 5 functionally conserved subsets of MSCs using scRNA-seq, demonstrating that hUC-MSCs possess enhanced immunomodulatory potential [159, 160]. Additionally, Zhang S et al. discovered two distinct subsets of MSCs in hUC-MSCs with variations in immune regulation and tissue differentiation functions [161]. The presence of these diverse subsets of stem cells contributes to the bidirectional regulation of the immune system [2] and may also account for the dual regulatory effect on apoptosis.
Conclusion
The great potential of MSCs in the treatment of various diseases is attracting more and more attention, which makes the research on the therapeutic mechanism of MSCs more and more in-depth. The regulation of apoptosis is an important part of the MSCs therapeutic mechanism. In general, MSCs have been shown to inhibit apoptosis and promote survival of various tissue cells. Moreover, this process involves three major apoptotic regulatory pathways and is closely related to autophagy, aging, and proliferation. However, in partial disease states, MSCs will also show the promotion of apoptosis in specific cells, such as lymphocytes and tumor cells. And these pro-apoptotic effects are generally considered to be part of the immunosuppressive effect of MSCs. These complex and orderly regulatory mechanisms constitute the homeostasis regulatory network of MSCs on tissue cells, which is the basis for MSCs to exert various therapeutic effects. However, the mechanism of MSCs regulating apoptosis still needs to be further explored. In particular, the complex regulatory network of MSCs on apoptosis, autophagy, aging, proliferation and survival of tissue cells deserves more attention. These studies will help to further understand the important role of MSCs in maintaining homeostasis.
References
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507.
Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–16.
Cuesta-Gomez N, Graham GJ, Campbell J. Chemokines and their receptors: predictors of the therapeutic potential of mesenchymal stromal cells. J Transl Med. 2021;19:156.
Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Extrinsic and Intrinsic mechanisms by which mesenchymal stem cells suppress the immune system. Ocul Surf. 2016;14:121–34.
Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21:974–82.
Bovin LF, Bendtzen K. Apoptosis-programmed cell death. Ugeskr Laeger. 1999;161:5778–82.
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62.
Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080.
Bao WD, Zhou XT, Zhou LT, Wang F, Yin X, Lu Y, et al. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19:e13235.
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98:813–80.
Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–5.
Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417.
Niu J, Yu F, Luo X, Chen S. Human umbilical cord mesenchymal stem cells improve premature ovarian failure through cell apoptosis of miR-100-5p/NOX4/NLRP3. Biomed Res Int. 2022;2022:3862122.
Wang Y, Zhang JW, Wang JW, Wang JL, Zhang SC, Ma RY, et al. BMSCs overexpressed ISL1 reduces the apoptosis of islet cells through ANLN carrying exosome, INHBA, and caffeine. Cell Mol Life Sci. 2022;79:538.
Song H, Li B, Guo R, He S, Peng Z, Qu J, et al. Hypoxic preconditioned aged BMSCs accelerates MI injury repair by modulating inflammation, oxidative stress and apoptosis. Biochem Biophys Res Commun. 2022;627:45–51.
Cheng T, Ding S, Liu S, Li Y, Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorate lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics. 2021;11:893–905.
Lee HK, Kim HS, Pyo M, Park EJ, Jang S, Jun HW, et al. Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL.Faslpr mice. Theranostics. 2020;10:10186–99.
Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55.
Raziyeva K, Smagulova A, Kim Y, Smagul S, Nurkesh A, Saparov A. Preconditioned and genetically modified stem cells for myocardial infarction treatment. Int J Mol Sci. 2020;21:7301.
Birtwistle L, Chen XM, Pollock C. Mesenchymal stem cell-derived extracellular vesicles to the rescue of renal injury. Int J Mol Sci. 2021;22:6596.
Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal stem cell-derived exosomes as new remedy for the treatment of neurocognitive disorders. Int J Mol Sci. 2021;22:1433.
Takahashi A, Yousif A, Hong L, Chefetz I. Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell. J Mol Med. 2021;99:637–50.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64.
Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–94.
Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77.
Burke PJ. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–70.
Vander HM, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–16.
Gu C, Du W, Chai M, Jin Z, Zhou Y, Guo P, et al. Human umbilical cord-derived mesenchymal stem cells affect urea synthesis and the cell apoptosis of human induced hepatocytes by secreting IL-6 in a serum-free co-culture system. Biotechnol J. 2022;17:e2100096.
Zhang Y, Li Y, Wang Q, Zheng D, Feng X, Zhao W, et al. Attenuation of hepatic ischemiareperfusion injury by adipose stem cellderived exosome treatment via ERK1/2 and GSK3beta signaling pathways. Int J Mol Med. 2022;49:13.
Xu TB, Li L, Luo XD, Lin H. BMSCs protect against liver injury via suppressing hepatocyte apoptosis and activating TGF-beta1/Bax singling pathway. Biomed Pharmacother. 2017;96:1395–402.
Rozier P, Maumus M, Maria A, Toupet K, Lai-Kee-Him J, Jorgensen C, et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021;121:102660.
Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST, et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248–66.
Shi X, Zhong X, Deng L, Wu X, Zhang P, Zhang X, et al. Mesenchymal stem cell-derived extracellular vesicle-enclosed microRNA-93 prevents hypoxic-ischemic brain damage in rats. Neuroscience. 2022;500:12–25.
Wu Z, Cheng S, Wang S, Li W, Liu J. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int Immunopharmacol. 2021;93:107389.
Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu Y, et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 2020;11:696.
Shologu N, Scully M, Laffey JG, O’Toole D. Human mesenchymal stem cell secretome from bone marrow or adipose-derived tissuesources for treatment of hypoxia-induced pulmonary epithelial injury. Int J Mol Sci. 2018;19:2996.
Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325:80–8.
Vianello F, Villanova F, Tisato V, Lymperi S, Ho KK, Gomes AR, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010;95:1081–9.
Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q, et al. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 2009;28:141.
Kuramoto Y, Fujita M, Takagi T, Takeda Y, Doe N, Yamahara K, et al. Early-phase administration of human amnion-derived stem cells ameliorates neurobehavioral deficits of intracerebral hemorrhage by suppressing local inflammation and apoptosis. J Neuroinflammation. 2022;19:48.
Zhou X, Chu X, Yuan H, Qiu J, Zhao C, Xin D, et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed Pharmacother. 2019;115:108818.
Su Y, Song X, Teng J, Zhou X, Dong Z, Li P, et al. Mesenchymal stem cells-derived extracellular vesicles carrying microRNA-17 inhibits macrophage apoptosis in lipopolysaccharide-induced sepsis. Int Immunopharmacol. 2021;95:107408.
Wang J, Qin Y, Mi X. The protective effects of bone marrow-derived mesenchymal stem cell (BMSC) on LPS-induced acute lung injury via TLR3-mediated IFNs, MAPK and NF-kappaB signaling pathways. Biomed Pharmacother. 2016;79:176–87.
Li B, Leung J, Chan L, Yiu WH, Li Y, Lok S, et al. Amelioration of endoplasmic reticulum stress by mesenchymal stem cells via hepatocyte growth factor/c-Met signaling in obesity-associated kidney injury. Stem Cells Transl Med. 2019;8:898–910.
Li Q, Song WJ, Ryu MO, Nam A, An JH, Ahn JO, et al. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates severe acute pancreatitis via ER stress downregulation in mice. Stem Cell Res Ther. 2018;9:255.
Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97.
He Y, Zhang D, Zeng Y, Ma J, Wang J, Guo H, et al. Bone marrow-derived mesenchymal stem cells protect islet grafts against endoplasmic reticulum stress-induced apoptosis during the early stage after transplantation. Stem Cells. 2018;36:1045–61.
Wang J, Hu K, Cai X, Yang B, He Q, Wang J, et al. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm Sin B. 2022;12:18–32.
Xiao X, Li W, Xu Z, Sun Z, Ye H, Wu Y, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells reduce lipopolysaccharide-induced spinal cord injury neuronal apoptosis by mediating miR-29b-3p/PTEN. Connect Tissue Res. 2022;63:634–49.
Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:290.
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36.
Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32.
Wen C, Lin L, Zou R, Lin F, Liu Y. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle. 2022;21:289–303.
Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11:260.
Geng Z, Chen H, Zou G, Yuan L, Liu P, Li B, et al. Human amniotic fluid mesenchymal stem cell-derived exosomes inhibit apoptosis in ovarian granulosa cell via miR-369-3p/YAF2/PDCD5/p53 Pathway. Oxid Med Cell Longev. 2022;2022:3695848.
Sun B, Ma Y, Wang F, Hu L, Sun Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10:360.
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163–77.
Wang Y, Shen Y. Exosomal miR-455-3p from BMMSCs prevents cardiac ischemia-reperfusion injury. Hum Exp Toxicol. 2022;41:774846548.
Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19:339–53.
Sherif IO, Sabry D, Abdel-Aziz A, Sarhan OM. The role of mesenchymal stem cells in chemotherapy-induced gonadotoxicity. Stem Cell Res Ther. 2018;9:196.
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34.
Karaoz E, Genc ZS, Demircan PC, Aksoy A, Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2010;1:e36.
Li X, Michaeloudes C, Zhang Y, Wiegman CH, Adcock IM, Lian Q, et al. Mesenchymal stem cells alleviate oxidative stress-induced mitochondrial dysfunction in the airways. J Allergy Clin Immunol. 2018;141:1634–45.
Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9:2017–35.
Hu Y, Tao R, Wang L, Chen L, Lin Z, Panayi AC, et al. Exosomes derived from bone mesenchymal stem cells alleviate compression-induced nucleus pulposus cell apoptosis by inhibiting oxidative stress. Oxid Med Cell Longev. 2021;2021:2310025.
Piao L, Huang Z, Inoue A, Kuzuya M, Cheng XW. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem Cell Res Ther. 2022;13:226.
Guo J, Wang R, Liu D. Bone marrow-derived mesenchymal stem cells ameliorate sepsis-induced acute kidney injury by promoting mitophagy of renal tubular epithelial cells via the SIRT1/Parkin axis. Front Endocrinol (Lausanne). 2021;12:639165.
Zheng J, Chen L, Lu T, Zhang Y, Sui X, Li Y, et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKalpha activation. Cell Death Dis. 2020;11:256.
Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95.
Li X, Xie X, Yu Z, Chen Y, Qu G, Yu H, et al. Bone marrow mesenchymal stem cells-derived conditioned medium protects cardiomyocytes from hypoxia/reoxygenation-induced injury through Notch2/mTOR/autophagy signaling. J Cell Physiol. 2019;234:18906–16.
Hao Y, Zhu G, Yu L, Ren Z, Zhang P, Zhu J, et al. Extracellular vesicles derived from mesenchymal stem cells confer protection against intervertebral disc degeneration through a microRNA-217-dependent mechanism. Osteoarthr Cartil. 2022;30:1455–67.
Li G, Song Y, Liao Z, Wang K, Luo R, Lu S, et al. Bone-derived mesenchymal stem cells alleviate compression-induced apoptosis of nucleus pulposus cells by N6 methyladenosine of autophagy. Cell Death Dis. 2020;11:103.
Piao C, Sang J, Kou Z, Wang Y, Liu T, Lu X, et al. Effects of exosomes derived from adipose-derived mesenchymal stem cells on pyroptosis and regeneration of injured liver. Int J Mol Sci. 2022;23:12065.
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang X, et al. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxid Med Cell Longev. 2021;2021:6219715.
Wang D, Xue H, Tan J, Liu P, Qiao C, Pang C, et al. Bone marrow mesenchymal stem cells-derived exosomes containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1 signalling to alleviate inflammatory bowel disease. Inflamm Res. 2022;71:833–46.
Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, et al. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728–42.
Pan L, Yan B, Zhang J, Zhao P, Jing Y, Yu J, et al. Mesenchymal stem cells-derived extracellular vesicles-shuttled microRNA-223-3p suppress lipopolysaccharide-induced cardiac inflammation, pyroptosis, and dysfunction. Int Immunopharmacol. 2022;110:108910.
Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol Med. 2021;27:91.
Zhao Y, Chen Y, Wang Z, Xu C, Qiao S, Liu T, et al. Bone marrow mesenchymal stem cell exosome attenuates inflammasome-related pyroptosis via delivering circ_003564 to improve the recovery of spinal cord injury. Mol Neurobiol. 2022;59:6771–89.
Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu J, et al. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9:12.
Del RD, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99:1765–817.
Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11:317.
Li Y, Zhou J, Zhang O, Wu X, Guan X, Xue Y, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. Int Immunopharmacol. 2020;80:106156.
Lee TL, Lai TC, Lin SR, Lin SW, Chen YC, Pu CM, et al. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics. 2021;11:3131–49.
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393.
Yan W, Lin C, Guo Y, Chen Y, Du Y, Lau WB, et al. N-cadherin overexpression mobilizes the protective effects of mesenchymal stromal cells against ischemic heart injury through a beta-catenin-dependent manner. Circ Res. 2020;126:857–74.
Alijani-Ghazyani Z, Sabzevari R, Roushandeh AM, Jahanian-Najafabadi A, Amiri F, Roudkenar MH. Transplantation of umbilical cord-derived mesenchymal stem cells overexpressing lipocalin 2 ameliorates ischemia-induced injury and reduces apoptotic death in a rat acute myocardial infarction model. Stem Cell Rev Rep. 2020;16:968–78.
Zhang Z, Zhu L, Feng P, Tan Y, Zhang B, Gao E, et al. C1q/tumor necrosis factor-related protein-3-engineered mesenchymal stromal cells attenuate cardiac impairment in mice with myocardial infarction. Cell Death Dis. 2019;10:530.
Shen H, Cui G, Li Y, Ye W, Sun Y, Zhang Z, et al. Follistatin-like 1 protects mesenchymal stem cells from hypoxic damage and enhances their therapeutic efficacy in a mouse myocardial infarction model. Stem Cell Res Ther. 2019;10:17.
Gong XH, Liu H, Wang SJ, Liang SW, Wang GG. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J Cell Physiol. 2019;234:13878–93.
Liang X, Ding Y, Zhang Y, Chai YH, He J, Chiu SM, et al. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015;6:e1765.
Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, et al. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Transl Med. 2012;1:759–69.
Zhang J, Lu Y, Mao Y, Yu Y, Wu T, Zhao W, et al. IFN-gamma enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Res Ther. 2022;13:333.
Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116:353–67.
Chen R, Cai X, Liu J, Bai B, Li X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018;215:31–42.
Zhang GW, Gu TX, Guan XY, Sun XJ, Qi X, Li XY, et al. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015;48:661–70.
Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89.
Zito G, Miceli V, Carcione C, Busa R, Bulati M, Gallo A, et al. Human amnion-derived mesenchymal stromal/stem cells pre-conditioning inhibits inflammation and apoptosis of immune and parenchymal cells in an in vitro model of liver ischemia/reperfusion. Cells. 2022;11:709.
Liu Y, Ren H, Wang J, Yang F, Li J, Zhou Y, et al. Prostaglandin E2 secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J. 2019;33:2514–25.
Huang B, Cheng X, Wang H, Huang W, la Ga HZ, Wang D, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14:45.
Tian Y, Wang J, Wang W, Ding Y, Sun Z, Zhang Q, et al. Mesenchymal stem cells improve mouse non-heart-beating liver graft survival by inhibiting Kupffer cell apoptosis via TLR4-ERK1/2-Fas/FasL-caspase3 pathway regulation. Stem Cell Res Ther. 2016;7:157.
Ezquer F, Bahamonde J, Huang YL, Ezquer M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res Ther. 2017;8:20.
Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9.
Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, et al. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235:3698–710.
Zhang ZH, Zhu W, Ren HZ, Zhao X, Wang S, Ma HC, et al. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res Ther. 2017;8:70.
Qin HH, Filippi C, Sun S, Lehec S, Dhawan A, Hughes RD. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res Ther. 2015;6:237.
He X, Li C, Yin H, Tan X, Yi J, Tian S, et al. Mesenchymal stem cells inhibited the apoptosis of alveolar epithelial cells caused by ARDS through CXCL12/CXCR4 axis. Bioengineered. 2022;13:9060–70.
Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68–76.
Xu B, Gan CX, Chen SS, Li JQ, Liu MZ, Guo GH. BMSC-derived exosomes alleviate smoke inhalation lung injury through blockade of the HMGB1/NF-kappaB pathway. Life Sci. 2020;257:118042.
Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17.
Mao GC, Gong CC, Wang Z, Sun MX, Pei ZP, Meng WQ, et al. BMSC-derived exosomes ameliorate sulfur mustard-induced acute lung injury by regulating the GPRC5A-YAP axis. Acta Pharmacol Sin. 2021;42:2082–93.
Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, et al. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17:560–70.
Chen Y, Zhang F, Wang D, Li L, Si H, Wang C, et al. Mesenchymal stem cells attenuate diabetic lung fibrosis via adjusting Sirt3-mediated stress responses in rats. Oxid Med Cell Longev. 2020;2020:8076105.
Chen S, Cui G, Peng C, Lavin MF, Sun X, Zhang E, et al. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9:110.
Cahill EF, Kennelly H, Carty F, Mahon BP, English K. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5:1307–18.
Cheng C, Chen X, Wang Y, Cheng W, Zuo X, Tang W, et al. MSCsderived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. 2021;27:67.
Li J, Li H, Cai S, Bai S, Cai H, Zhang X. CD157 in bone marrow mesenchymal stem cells mediates mitochondrial production and transfer to improve neuronal apoptosis and functional recovery after spinal cord injury. Stem Cell Res Ther. 2021;12:289.
Qin C, Bai L, Li Y, Wang K. The functional mechanism of bone marrow-derived mesenchymal stem cells in the treatment of animal models with Alzheimer’s disease: crosstalk between autophagy and apoptosis. Stem Cell Res Ther. 2022;13:90.
Luo Q, Tang Y, Jiang Z, Bao H, Fu Q, Zhang H. hUCMSCs reduce theca interstitial cells apoptosis and restore ovarian function in premature ovarian insufficiency rats through regulating NR4A1-mediated mitochondrial mechanisms. Reprod Biol Endocrinol. 2022;20:125.
Zhirong Z, Li H, Yiqun H, Chunyang H, Lichen Z, Zhen T, et al. Enhancing or inhibiting apoptosis? The effects of ucMSC-Ex in the treatment of different degrees of traumatic pancreatitis. Apoptosis. 2022;27:521–30.
Yu XJ, Liu QK, Lu R, Wang SX, Xu HR, Wang YG, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicles carrying circ_0050205 attenuate intervertebral disc degeneration. Oxid Med Cell Longev. 2022;2022:8983667.
Wang Z, Yan K, Ge G, Zhang D, Bai J, Guo X, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37:85–96.
Cheng H, Ding J, Tang G, Huang A, Gao L, Yang J, et al. Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway. Mol Med. 2021;27:128.
Jiang S, Mo C, Guo S, Zhuang J, Huang B, Mao X. Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J Exp Clin Canc Res. 2019;38:495.
Yoon N, Park MS, Shigemoto T, Peltier G, Lee RH. Activated human mesenchymal stem/stromal cells suppress metastatic features of MDA-MB-231 cells by secreting IFN-β. Cell Death Dis. 2016;7:e2191.
Atsuta I, Liu S, Miura Y, Akiyama K, Chen C, An Y, et al. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther. 2013;4:111.
Lu L, Chen G, Yang J, Ma Z, Yang Y, Hu Y, et al. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed Pharmacother. 2019;112:108625.
Chen YC, Lan YW, Huang SM, Yen CC, Chen W, Wu WJ, et al. Human amniotic fluid mesenchymal stem cells attenuate pancreatic cancer cell proliferation and tumor growth in an orthotopic xenograft mouse model. Stem Cell Res Ther. 2022;13:235.
Serhal R, Saliba N, Hilal G, Moussa M, Hassan GS, Atat OE, et al. Effect of adipose-derived mesenchymal stem cells on hepatocellular carcinoma: In vitro inhibition of carcinogenesis. World J Gastroenterol. 2019;25:567–83.
Lin DH, Biswas A, Choolani M, Fong CY, Bongso A. Induction of immunogenic cell death in lymphoma cells by wharton’s jelly mesenchymal stem cell conditioned medium. Stem Cell Rev Rep. 2017;13:801–16.
Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–604.
Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed Death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35:766–76.
Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(-) regulatory T cells. Cell Mol Immunol. 2015;12:708–18.
Jang YO, Jun BG, Baik SK, Kim MY, Kwon SO. Inhibition of hepatic stellate cells by bone marrow-derived mesenchymal stem cells in hepatic fibrosis. Clin Mol Hepatol. 2015;21:141–9.
Zhang LT, Peng XB, Fang XQ, Li JF, Chen H, Mao XR. Human umbilical cord mesenchymal stem cells inhibit proliferation of hepatic stellate cells in vitro. Int J Mol Med. 2018;41:2545–52.
Zhang Y, Tu B, Sha Q, Qian J. Bone marrow mesenchymal stem cells-derived exosomes suppress miRNA-5189-3p to increase fibroblast-like synoviocyte apoptosis via the BATF2/JAK2/STAT3 signaling pathway. Bioengineered. 2022;13:6767–80.
Li Q, Huang P, Chen W, Bi J. Mechanism of bone marrow mesenchymal stem cells secreting miR-26a exosomes affecting high glucose-induced skin fibroblasts function by regulating TLR4/NF-kappaB signaling. Inflamm Res. 2021;70:811–21.
Zhang F, Peng W, Wang T, Zhang J, Dong W, Wang C, et al. Lnc Tmem235 promotes repair of early steroid-induced osteonecrosis of the femoral head by inhibiting hypoxia-induced apoptosis of BMSCs. Exp Mol Med. 2022;54:1991–2006.
Yang W, Zhang S, Ou T, Jiang H, Jia D, Qi Z, et al. Interleukin-11 regulates the fate of adipose-derived mesenchymal stem cells via STAT3 signalling pathways. Cell Prolif. 2020;53:e12771.
Jia B, Jiang Y, Yao Y, Xu Y, Wang Y, Li T. Baicalin attenuates dexamethasone-induced apoptosis of bone marrow mesenchymal stem cells by activating the hedgehog signaling pathway. Chin Med J-Peking. 2023;136:1839–47.
Duan W, Zou H, Zang N, Ma D, Yang B, Zhu L. Metformin increases bone marrow adipose tissue by promoting mesenchymal stromal cells apoptosis. Aging. 2023;15:542–52.
Sun Y, Xu H, Tan B, Yi Q, Liu H, Chen T, et al. Andrographolide protects bone marrow mesenchymal stem cells against glucose and serum deprivation under hypoxia via the NRF2 signaling pathway. Stem Cell Res Ther. 2022;13:326.
Jiang D, Tuo L, Bai X, Bing W, Qu Q, Zhao X, et al. Prostaglandin E1 reduces apoptosis and improves the homing of mesenchymal stem cells in pulmonary arterial hypertension by regulating hypoxia-inducible factor 1 alpha. Stem Cell Res Ther. 2022;13:316.
Wang T, Zhang F, Peng W, Wang L, Zhang J, Dong W, et al. Overexpression of NMNAT3 improves mitochondrial function and enhances antioxidative stress capacity of bone marrow mesenchymal stem cells via the NAD+-Sirt3 pathway. Biosci Rep. 2022;42:BSR20211005.
Li M, Cong R, Yang L, Yang L, Zhang Y, Fu Q. A novel lncRNA LNC_000052 leads to the dysfunction of osteoporotic BMSCs via the miR-96-5p–PIK3R1 axis. Cell Death Dis. 2020;11:795.
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134:2648–57.
Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9:eaam7828.
Tang H, Luo H, Zhang Z, Yang D. Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential. Cells. 2022;11:3879.
Giacomini C, Granéli C, Hicks R, Dazzi F. The critical role of apoptosis in mesenchymal stromal cell therapeutics and implications in homeostasis and normal tissue repair. Cell Mol Immunol. 2023;20:570–82.
Cheung TS, Giacomini C, Cereda M, Avivar-Valderas A, Capece D, Bertolino GM, et al. Apoptosis in mesenchymal stromal cells activates an immunosuppressive secretome predicting clinical response in Crohn’s disease. Mol Ther. 2023;31:3531–44.
Ghahremani PM, Soudi S, Ghanbarian H, Bolandi Z, Namaki S, Hashemi SM. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. 2018;212:203–12.
Li Y, Shen S, Shao T, Jin M, Fan D, Lin A, et al. Mesenchymal stem cells attenuate liver fibrosis by targeting Ly6Chi/lo macrophages through activating the cytokine-paracrine and apoptotic pathways. Cell Death Discov. 2021;7:239.
Vogel S, Trapp T, Börger V, Peters C, Lakbir D, Dilloo D, et al. Hepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and cardiomyocytic cells. Cell Mol Life Sci. 2010;67:295–303.
Vogel S, Borger V, Peters C, Forster M, Liebfried P, Metzger K, et al. Necrotic cell-derived high mobility group box 1 attracts antigen-presenting cells but inhibits hepatocyte growth factor-mediated tropism of mesenchymal stem cells for apoptotic cell death. Cell Death Differ. 2015;22:1219–30.
Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018;28:918–33.
Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. Ebiomedicine. 2019;45:341–50.
Cuerquis J, Romieu-Mourez R, Francois M, Routy JP, Young YK, Zhao J, et al. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy. 2014;16:191–202.
Zhang HT, Zha ZG, Cao JH, Liang ZJ, Wu H, He MT, et al. Apigenin accelerates lipopolysaccharide induced apoptosis in mesenchymal stem cells through suppressing vitamin D receptor expression. Chin Med J (Engl). 2011;124:3537–45.
Sun C, Wang L, Wang H, Huang T, Yao W, Li J, et al. Single-cell RNA-seq highlights heterogeneity in human primary Wharton’s jelly mesenchymal stem/stromal cells cultured in vitro. Stem Cell Res Ther. 2020;11:149.
Wang Z, Chai C, Wang R, Feng Y, Huang L, Zhang Y, et al. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin Transl Med. 2021;11:e650.
Zhang S, Wang JY, Li B, Yin F, Liu H. Single-cell transcriptome analysis of uncultured human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2021;12:25.
Ebrahim N, Al SH, Mostafa O, Hassouna A, Abdulsamea S, Abd EAME, et al. Prophylactic evidence of MSCs-derived exosomes in doxorubicin/trastuzumab-induced cardiotoxicity: beyond mechanistic target of NRG-1/Erb signaling pathway. Int J Mol Sci. 2022;23:5967.
Hong L, Yan L, Xin Z, Hao J, Liu W, Wang S, et al. Protective effects of human umbilical cord mesenchymal stem cell-derived conditioned medium on ovarian damage. J Mol Cell Biol. 2020;12:372–85.
Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, Liu QY, et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 2019;10:247.
Nie Y, Han BM, Liu XB, Yang JJ, Wang F, Cong XF, et al. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci. 2011;7:762–8.
Berlier JL, Rigutto S, Dalla VA, Lechanteur J, Soyfoo MS, Gangji V, et al. Adenosine triphosphate prevents serum deprivation-induced apoptosis in human mesenchymal stem cells via activation of the MAPK signaling pathways. Stem Cells. 2015;33:211–8.
Lee HJ, Jung YH, Choi GE, Ko SH, Lee S, Lee SH, et al. BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol. 2017;13:426–43.
Wang T, Xie Z, Wang L, Luo H, Zhang J, Dong W, et al. LncAABR07053481 inhibits bone marrow mesenchymal stem cell apoptosis and promotes repair following steroid-induced avascular necrosis. Commun Biol. 2023;6:365.
Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2:845–54.
Xu J, Huang Z, Lin L, Fu M, Gao Y, Shen Y, et al. miR-210 over-expression enhances mesenchymal stem cell survival in an oxidative stress environment through antioxidation and c-Met pathway activation. Sci China Life Sci. 2014;57:989–97.
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S, et al. Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt–Sfrp2 pathways. Free Radic Bio Med. 2014;77:363–75.
Jing H, Sun X, Li M, Peng J, Gu X, Xiong J. Exogenous melatonin activating nuclear factor E2-related factor 2 (Nrf2) pathway via melatonin receptor to reduce oxidative stress and apoptosis in antler mesenchymal stem cells. Molecules. 2022;27:2515.
Yang C, Guo X, Yue Y, Wang Y, Jin X. Astaxanthin promotes the survival of adipose-derived stem cells by alleviating oxidative stress via activating the Nrf2 Signaling Pathway. Int J Mol Sci. 2023;24:3850.
Suwanmanee G, Tantrawatpan C, Kheolamai P, Paraoan L, Manochantr S. Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway. Sci Rep. 2023;13:22974.
Gao B, Han Y, Wang L, Lin Y, Sun Z, Lu W, et al. Eicosapentaenoic acid attenuates dexamethasome-induced apoptosis by inducing adaptive autophagy via GPR120 in murine bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2016;7:e2235.
Lu C, Chen J, Zhou G, Wu S, Guan Y, Yuan C. Multimolecular complex of Par-4 and E2F1 binding to Smac promoter contributes to glutamate-induced apoptosis in human- bone mesenchymal stem cells. Nucleic Acids Res. 2008;36:5021–32.
Ghali O, Chauveau C, Hardouin P, Broux O, Devedjian J. TNF-α‘s effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J Bone Min Res. 2010;25:1616–26.
Kim JY, Shin KK, Lee AL, Kim YS, Park HJ, Park YK, et al. MicroRNA-302 induces proliferation and inhibits oxidant-induced cell death in human adipose tissue-derived mesenchymal stem cells. Cell Death Dis. 2014;5:e1385.
Zhu M, He X, Wang X, Qiu W, Xing W, Guo W, et al. Complement C5a induces mesenchymal stem cell apoptosis during the progression of chronic diabetic complications. Diabetologia. 2017;60:1822–33.
Yin Y, Chen F, Li J, Yang J, Li Q, Jin P. AURKA enhances autophagy of adipose derived stem cells to promote diabetic wound repair via targeting FOXO3a. J Investig Dermatol. 2020;140:1639–49.
Li L, Sun Y, Zhang N, Qiu X, Wang L, Luo Q. By regulating miR-182-5p/BCL10/CYCS, sufentanil reduces the apoptosis of umbilical cord mesenchymal stem cells caused by ropivacaine. Biosci Trends. 2019;13:49–57.
Liu Z, Li T, Zhu F, Deng SN, Li X, He Y. Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis. 2019;10:227.
Zhang F, Yan Y, Peng W, Wang L, Wang T, Xie Z, et al. PARK7 promotes repair in early steroid-induced osteonecrosis of the femoral head by enhancing resistance to stress-induced apoptosis in bone marrow mesenchymal stem cells via regulation of the Nrf2 signaling pathway. Cell Death Dis. 2021;12:940.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 82372529; 82303759; 32100643); National Defense Basic Scientific Research Project (No. 2022-JCJQ-ZD-224-12).
Author information
Authors and Affiliations
Contributions
ZC, MWY and YY - draft preparation; ZQZ – figure preparation; ZC and XA – table preparation; LG and XX – final manuscript preparation; XWX – manuscript revision. all authors read and accepted the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by: Professor Yufang Shi
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Z., Xia, X., Yao, M. et al. The dual role of mesenchymal stem cells in apoptosis regulation. Cell Death Dis 15, 250 (2024). https://doi.org/10.1038/s41419-024-06620-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06620-x