Abstract
Skeletal muscle is the largest metabolic organ in the body, and its metabolic flexibility is essential for maintaining systemic energy homeostasis. Metabolic inflexibility in muscles is a dominant cause of various metabolic disorders, impeding muscle development. In our previous study, we found lncRNA ZFP36L2-AS (for “ZFP36L2-antisense transcript”) is specifically enriched in skeletal muscle. Here, we report that ZFP36L2-AS is upregulated during myogenic differentiation, and highly expressed in breast and leg muscle. In vitro, ZFP36L2-AS inhibits myoblast proliferation but promotes myoblast differentiation. In vivo, ZFP36L2-AS facilitates intramuscular fat deposition, as well as activates fast-twitch muscle phenotype and induces muscle atrophy. Mechanistically, ZFP36L2-AS interacts with acetyl-CoA carboxylase alpha (ACACA) and pyruvate carboxylase (PC) to induce ACACA dephosphorylation and damaged PC protein stability, thus modulating muscle metabolism. Meanwhile, ZFP36L2-AS can activate ACACA to reduce acetyl-CoA content, which enhances the inhibition of PC activity. Our findings present a novel model about the regulation of lncRNA on muscle metabolism.
Similar content being viewed by others
Introduction
As the largest tissue that comprises about 40% of the total body mass, skeletal muscle is a major player in regulating energy homeostasis and obesity progression [1,2,3]. A key metabolic feature of skeletal muscle is its plasticity, which is able to adjust fuel oxidation to fuel availability, called ‘metabolic flexibility’ [4, 5]. It’s well known that the metabolic regulation of skeletal muscle is pivotal for health and development, and loss of this flexibility is tightly associated with metabolic disorders such as obesity and muscle wasting [4, 6,7,8].
The maintenance of skeletal muscle mass is finely regulated by protein synthesis and catabolism [9]. Muscle atrophy refers to a decrease in muscle mass and fiber size and is characterized by enhanced protein degradation [10]. Muscle atrophy leading to muscle wasting seriously restricts animal development. Recently, muscle wasting has attracted many researchers’ attention, however, the molecular mechanisms that govern muscle atrophy remain largely unknown.
Protein-encoding genes only account for a small portion (2%) of the genome, and yet 70–90% of the genome is transcribed into long noncoding RNAs (lncRNAs) at some point during development [11]. LncRNAs are a new class of regulatory RNAs, commonly defined as transcribed RNAs of more than 200 nucleotides with low coding potential, are widely involved in gene expression regulation at the transcription, translation and epigenetic levels [12,13,14,15,16]. Although only a small number of functional lncRNAs have been well characterized to date, they seem to control major biological processes impacting skeletal muscle development and muscle disorders [17,18,19,20].
White recessive rock (WRR) is a hypertrophic broiler chicken with a fast growth rate, which exhibits a different growth performance from Xinghua (XH) chicken (a lean Chinese native breed with a slow growth rate) [21, 22]. In our previous RNA-seq study (accession number GSE58755), we found lncRNA TCONS_00067025 (named ZFP36 ring finger protein like 2 [ZFP36L2]-antisense transcript [ZFP36L2-AS]) differentially expressed between WRR (a fast growth rate broiler chicken) and XH chicken (a slow growth rate Chinese native breed) [22]. In the current study, functional studies demonstrated that ZFP36L2-AS inhibits myoblast proliferation but promotes myogenic differentiation in vitro. In vivo, ZFP36L2-AS represses fatty acid oxidation to facilitate intramuscular fat deposition, as well as activates fast-twitch muscle phenotype and induces muscle atrophy. Further mechanistic investigation revealed that ZFP36L2-AS interacts with acetyl-CoA carboxylase alpha (ACACA) and pyruvate carboxylase (PC) to induce ACACA activation and inhibit PC activity. Altogether, our studies uncover a functional lncRNA that modulates skeletal muscle development.
Results
ZFP36L2-AS is a novel lncRNA associated with skeletal muscle development
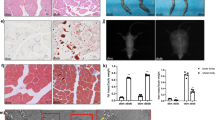
Our previous RNA-seq study found a novel lncRNA (ZFP36L2-AS) was highly expressed in fast growth rate broilers (Fig. 1A, B) [22], implied that ZFP36L2-AS is probably associated with skeletal muscle development. To obtain the full-length of ZFP36L2-AS, 5’ and 3’ ends of this lncRNA were determined by RACE system (Fig. 1C). The Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) showed that ZFP36L2-AS was an antisense transcript of ZFP36L2 with 3,465 nt long, located at chromosome 3 from 25,089,365 to 25,092,829, and mainly conserved in Aves (Supplementary Fig. 1A and Table 1). ZFP36L2-AS was upregulated during myogenic differentiation, and highly expressed in breast and leg muscle (Fig. 1D, E). Compared with other muscle-resident cells, ZFP36L2-AS was highly expressed in myoblasts (Supplementary Fig. 1B). Furthermore, cell-fractionation assays demonstrated that ZFP36L2-AS was present both in the cytoplasm and nucleus of chicken primary myoblast (CPM) (Fig. 1F, G). In order to verify the coding potential of ZFP36L2-AS, we further cloned 3xFLAG epitope tag in-frame with the C terminus of thirteen potential ORFs of ZFP36L2-AS. Crucially, western blot analysis indicated that ZFP36L2-AS was an lncRNA without protein-encoding potential (Fig. 1H).
A, B Relative ZFP36L2-AS expression in breast muscles of 7-week-old Xinghua (XH) chicken and White recessive rock (WRR) detected by RNA-seq (A) and qPCR (B). (C) Results of ZFP36L2-AS 3’ RACE and 5’ RACE. D Relative ZFP36L2-AS expression during CPM proliferation and differentiation. E Tissue expression profiles of ZFP36L2-AS. The horizontal axis and vertical axis indicate different tissues and their relative expression values, respectively. F, G The distribution of ZFP36L2-AS in the cytoplasm and nuclei of chicken primary myoblast (CPM) was determined by qPCR (F) and semi-qPCR (G). GAPDH and U6 serve as cytoplasmic and nuclear localization controls, respectively. H Western blot analysis of the coding ability of ZFP36L2-AS. The potential ORFs of ZFP36L2-AS were cloned into the pcDNA3.1-3xFlag-C vector. CPMs transfected with β-actin were used as a positive control (PC) and untransfected CPMs were used as a negative control (NC). Results are presented as mean ± SEM. In panels (A, B), statistical significance of differences between means was assessed using independent sample t-test. (*P < 0.05; **P < 0.01).
ZFP36L2-AS inhibits myoblast proliferation but promotes myogenic differentiation
Given that ZFP36L2-AS was decreased in myoblast proliferation and upregulated during myogenic differentiation (Fig. 1D), we performed overexpression and inhibition experiments to assess its effect in proliferation and differentiation of myoblast (Fig. 2A and Supplementary Fig. 2A). The 5-ethynyl-2′-deoxyuridine (EdU) staining and cell counting kit-8 (CCK-8) assay demonstrated that ZFP36L2-AS interference significantly increased EdU incorporation and promoted myoblast proliferation, whereas ZFP36L2-AS overexpression significantly inhibited the proliferation of myoblast (Fig. 2B–D and Supplementary Fig. 2B–D). Flow cytometric analysis showed that ZFP36L2-AS inhibition significantly reduced the number of cells that progressed to G0/G1 and increased the number of S phase cells (Fig. 2E). Conversely, ZFP36L2-AS overexpression resulted in a larger number of G0/G1 and fewer S phase cells (Supplementary Fig. 2E). Furthermore, inhibition of ZFP36L2-AS increased the expression of cell cycle-promoting genes such as CCNB2 and CCND1, while reduced cell cycle-inhibiting genes like CDKN1A and CDKN1B (Fig. 2F). And the opposite result was observed with ZFP36L2-AS overexpression (Supplementary Fig. 2F).
A Relative ZFP36L2-AS expression with ZFP36L2-AS interference in vitro. B Proliferation of transfected CPMs was assessed by 5-ethynyl-2’-deoxyuridine (EdU) incorporation. C Proliferation rate of myoblasts after interference of ZFP36L2-AS. D CCK-8 assays were performed in CPMs with ZFP36L2-AS interference. E Cell cycle analysis of myoblasts after interference of ZFP36L2-AS. F Relative mRNA levels of several cell cycle genes with ZFP36L2-AS interference. G–I MyHC immunostaining (G), myotube area (%) (H), and myoblast fusion index (I) of CPMs transduced with ZFP36L2-AS interference. Cells were differentiated for 72 h after transfection. The nuclei were visualized with 4′,6-diamidino-2phenylindole (DAPI). J, K Relative mRNA (J) and protein (K) expression levels of myoblast differentiation marker genes from si-ASO-ZFP36L2-AS transfected CPMs. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to β-Tubulin. Data are expressed as a fold-change relative to the control. Results are shown as mean ± SEM. In panels (A, C–F, and H–J), statistical significance of differences between means was assessed using independent sample t-test. (*P < 0.05; **P < 0.01; N.S. no significant difference).
To further test whether ZFP36L2-AS functions in myogenic differentiation, immunofluorescence staining was performed after overexpression and inhibition of ZFP36L2-AS. ZFP36L2-AS inhibition significantly repressed myoblast differentiation and decreased the total areas of myotubes, while myotube formation was facilitated with ZFP36L2-AS overexpression (Fig. 2G–I and Supplementary Fig. 2G–I). In addition, the expressions level of myoblast differentiation marker genes, including MYOD, MYOG, and MyHC were significantly downregulated with ZFP36L2-AS interference (Fig. 2J, K). Conversely, overexpression of ZFP36L2-AS promoted their expression (Supplementary Fig. 2J, K).
ZFP36L2-AS decreases cellular respiration, fatty acid oxidation, and TCA cycle metabolites in skeletal muscle
Cellular mitochondrial activities including oxygen consumption rate (OCR), basal and maximal mitochondrial respiration, and adenosine triphosphate (ATP) production were elevated with ZFP36L2-AS interference, whereas ZFP36L2-AS overexpression facilitated glycolysis (Fig. 3A–D and Supplementary Fig. 3A–D), indicating that ZFP36L2-AS may be involved in cellular energy metabolism. Meanwhile, inhibition of ZFP36L2-AS increased ATP content in myoblast, while ATP content was decreased with ZFP36L2-AS overexpression in myoblasts (Fig. 3E and Supplementary Fig. 3E). Given that ZFP36L2-AS regulates cellular metabolism, we further detected the cellular ATP content in satellite cells after inhibition and overexpression of ZFP36L2-AS to explore whether ZFP36L2-AS plays a metabolic regulatory role in satellite cells similar to that in myoblasts. The results shown that ZFP36L2-AS did not regulate cellular ATP content in satellite cells (Supplementary Fig. 4A, B), suggesting the function of ZFP36L2-AS in muscle metabolism mainly depends on its expression in myoblasts. To investigate the potential roles of ZFP36L2-AS in vivo, the gastrocnemius of 1day-old chick was injected with adenovirus-mediated ZFP36L2-AS overexpression (Adv-ZFP36L2-AS) or lentivirus-mediated ZFP36L2-AS knockdown (Lv-shZFP36L2-AS) (Fig. 3F and Supplementary Fig. 3F). ZFP36L2-AS knockdown increased mitochondrial DNA content, which was potentially contribute to the acceleration of fatty acid oxidation (FAO) (Fig. 3G, H). In contrast, mitochondrial DNA content and fatty acid β-oxidation were reduced after overexpression of ZFP36L2-AS (Supplementary Fig. 3G, H). Besides, knockdown of ZFP36L2-AS upregulated the expression of FAO-related gene like CPT1, but downregulated key genes involved in fatty acid synthesis (such as FASN, MCAT and OXSM) and reduced intramuscular free fatty acid (FFA) and triglyceride (TG) content (Fig. 3I–K). Meanwhile, opposite results were showed with ZFP36L2-AS overexpression (Supplementary Fig. 3I–K).
A, B Oxygen consumption rate (OCR) (A), and basal respiration, maximal respiration, and ATP production (B) of myoblasts after interference of ZFP36L2-AS. (C, D) Extracellular acidification rate (ECAR) (C), and glycolysis and glycolytic capacity (D) of myoblasts with ZFP36L2-AS interference. E Relative cellular adenosine triphosphate (ATP) content with ZFP36L2-AS interference in CPMs. F Relative ZFP36L2-AS expression in gastrocnemius after infected with lentivirus-mediated ZFP36L2-AS knockdown (Lv-shZFP36L2-AS) or negative control (Lv-NC). G Relative mitochondrial DNA (mtDNA) content in ZFP36L2-AS knockdown gastrocnemius. (H) Relative fatty acid β-oxidation rate in gastrocnemius with ZFP36L2-AS knockdown. I, J Relative mRNA (I) and protein (J) expression levels of fatty acid oxidation or synthesis related-genes after infected with the indicated lentivirus. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to β-Tubulin. Data are expressed as a fold-change relative to the control. K Relative free fatty acid (FFA) and triglyceride (TG) content in gastrocnemius with ZFP36L2-AS knockdown. Results are presented as mean ± SEM. In panels (B, D–I, and K), statistical significance of differences between means was assessed using independent sample t-test. (*P < 0.05; **P < 0.01).
Excessive lipid storage is often accompanied by changes in muscle metabolism [23,24,25,26]. To further study the regulation of ZFP36L2-AS on muscle metabolism, a comparative metabolome analysis was performed with ZFP36L2-AS knockdown in gastrocnemius. Hierarchical clustering analysis (HCA) based on metabolite levels showed that several tricarboxylic acid cycle (TCA cycle) metabolites actually accumulate in ZFP36L2-AS knockdown gastrocnemius (Fig. 4A, B and Supplementary Table 2). On the contrary, ZFP36L2-AS knockdown significantly reduced glycolytic metabolites such as Fructose 1,6-bisphosphate and Dihydroxyacetone phosphate (Fig. 4A, B and Supplementary Table 2). Altogether, our results indicated that ZFP36L2-AS impairs mitochondrial respiration and FAO, leading to the accumulation of lipid metabolites, which elevates glycolysis as compensatory responses (Fig. 4C).
A Hierarchical clustering analysis (HCA) of metabolites in gastrocnemius after infected with Lv-shZFP36L2-AS or Lv-NC. The colors indicate the relative levels in lncRNA ZFP36L2-AS knockdown or control group. B Relative metabolite content of glycolysis and tricarboxylic acid (TCA) cycle in gastrocnemius with ZFP36L2-AS knockdown. C Schematic diagram of metabolic pathways of glycolysis and TCA cycle affected by ZFP36L2-AS knockdown in the gastrocnemius. Upregulated metabolites are shown in red, and the downregulated metabolites are shown in green. In panel (C), results are shown as mean ± SEM, statistical significance of differences between means was assessed using independent sample t-test. (*P < 0.05; **P < 0.01).
ZFP36L2-AS activates a fast-twitch gene expression profile concurrent with muscle atrophy
Skeletal muscle is comprised of heterogeneous myofibers that differ in their physiological and metabolic parameters [27]. Compared with fast-twitch (type II; glycolytic) myofibers, slow-twitch (type I; oxidative) myofibers have more myoglobin, more mitochondria, and higher activity of oxidative metabolic enzymes [28, 29]. In response to environmental demands, skeletal muscle can remodel by activating signaling pathways to reprogram gene expression to sustain muscle performance [27]. Given that knockdown of ZFP36L2-AS reduced the accumulation of glycolytic metabolites and upregulated oxidative metabolism in gastrocnemius (Fig. 4B, C), ZFP36L2-AS may function in the transformation of myofiber type by modulating muscle metabolism. As expected, glycogen content was increased and expression of glycogenolytic and glycolytic genes was downregulated with ZFP36L2-AS knockdown (Fig. 5A, B). Inversely, overexpression of ZFP36L2-AS reduced the accumulation of glycogen, as well as promoted expression of glycogenolytic and glycolytic genes (Supplementary Fig. 5A, B). ZFP36L2-AS knockdown suppressed the activity of lactic dehydrogenase (LDH) and enhanced the activity of succinate dehydrogenase (SDH), whereas ZFP36L2-AS overexpression elevated glycolytic capacity and decrease oxidative capacity of skeletal muscle (Fig. 5C and Supplementary Fig. 5C). More importantly, immunohistochemical results showed that ZFP36L2-AS knockdown suppressed MYH1A/fast-twitch protein level and promoted the expression level of MYH7B/slow-twitch protein (Fig. 5D, E). The expressions of multiple fast-twitch myofiber genes such as SOX6, TNNC2 and TNNT3 were significantly promoted, while slow-twitch myofiber genes like TNNC1, TNNI1 and TNNT1 were inhibited with ZFP36L2-AS knockdown (Fig. 5F). On the contrary, ZFP36L2-AS overexpression upregulated fast-twitch protein level and expression of fast-twitch myofiber genes, drove the transformation of slow-twitch to fast-twitch myofibers (Supplementary Fig. 5D–F).
A Relative glycogen content in gastrocnemius with ZFP36L2-AS knockdown. B Relative mRNA expression levels of glycogenolytic and glycolytic genes in gastrocnemius after infected with the indicated lentivirus. (C) Relative enzymes activity of lactic dehydrogenase (LDH) and succinate dehydrogenase (SDH) in gastrocnemius infected with ZFP36L2-AS knockdown. D, E Immunohistochemistry analysis of MYH1/MYH7 (D) and MYH1/MYH7 protein content (E) of gastrocnemius after ZFP36L2-AS knockdown. (F) Relative mRNA expression levels of several fast-/slow-twitch myofiber genes with ZFP36L2-AS knockdown. G Relative gastrocnemius muscle weight after infected with the indicated lentivirus. H, I H&E staining (H) and frequency distribution of fiber cross-section area (CSA) (I) of transverse sections of gastrocnemius with ZFP36L2-AS knockdown. J Relative mRNA expression of the atrophy and autophagy-related genes in gastrocnemius after infected with the indicated lentivirus. K The protein expression levels of mTOR signaling after ZFP36L2-AS knockdown. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to β-Tubulin. Data are expressed as a fold-change relative to the control. Results are presented as mean ± SEM. In panels (A–C, E–G, and J), statistical significance of differences between means was assessed using independent sample t-test. (*P < 0.05; **P < 0.01).
Muscle remodeling can also affect muscle mass; this is regulated by anabolic and catabolic signaling pathways, which induce muscle hypertrophy and muscle atrophy, respectively [30]. Here, ZFP36L2-AS knockdown increased muscle mass and elevated the proportion of large myofiber (> 200 μm2) (Fig. 5G–I). Conversely, gastrocnemius mass was reduced and proportion of small myofiber (< 200 μm2) was increased with overexpression of ZFP36L2-AS (Supplementary Fig. 5G–I), suggesting ZFP36L2-AS is involved in muscle atrophy. Mammalian target of rapamycin (mTOR) is a master growth regulator that senses and integrates diverse nutritional and environmental cues, can resist muscle atrophy by inhibiting proteasomal degradation and autophagy [30,31,32]. To further explore the regulatory mechanism of ZFP36L2-AS on muscle atrophy, we assessed the mTOR signaling after ZFP36L2-AS overexpression and knockdown. ZFP36L2-AS knockdown facilitated Ser2488 phosphorylation of mTOR, thus inactivating ubiquitin-proteasome system (UPS) and autophagy-lysosomal system, while the mTOR signaling was inhibited with ZFP36L2-AS overexpression (Fig. 5J, K and Supplementary Fig. 5J, K), indicating that ZFP36L2-AS induces muscle atrophy by inhibiting the mTOR signaling.
ZFP36L2-AS interacts with ACACA and PC
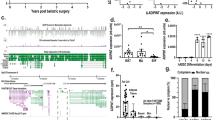
Molecular decoy is one of the main molecular mechanisms for lncRNA to function. It refers to that lncRNA directly binds to RNA or protein molecules, thereby activating or blocking the role and signal pathway of these molecule [33]. To elucidate the mechanism by which ZFP36L2-AS regulates skeletal muscle development, we attempted to identify its endogenous binding proteins by performing RNA pull-down coupled to mass spectrometry. Compared with the ZFP36L2-AS antisense group, a total of 141 proteins (protein score ≥ 19) were identified specifically bind to ZFP36L2-AS sense transcript (Supplementary Table 3). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis found that these RNA binding proteins (RBPs) were mainly enriched in biological processes such as cellular process, metabolic process, cellular component organization or biogenesis, and biological regulation, as well as participated in biological processes including metabolic pathways, carbon metabolism, TCA cycle, pyruvate metabolism, glycolysis/gluconeogenesis and so on (Supplementary Fig. 6A, B). Acetyl-CoA carboxylase alpha (ACACA) and pyruvate carboxylase (PC) are members of biotin-dependent carboxylase, which are known to be widely involved in metabolic regulation [34,35,36,37], were found specifically bind to ZFP36L2-AS (Supplementary Table 3). To corroborate this result, we performed western blot analysis of RNA pull-down samples, which validated the interaction of ZFP36L2-AS with ACACA and PC protein (Fig. 6A). Moreover, the specificity of these interactions was also verified with RNA immunoprecipitation (RIP) (Fig. 6B).
A, B lncRNA ZFP36L2-AS interacts with ACACA and PC protein were determined by biotin-labeled RNA pulldown (A) and RIP (B). C The interaction of full-length and truncated ZFP36L2-AS with ACACA and PC protein was determined by RNA pulldown. D–G The protein expression levels of ACACA and phosphorylated ACACA (D, F), and relative ACACA activity (E and G) after ZFP36L2-AS overexpression or knockdown in vitro and in vivo. H–K The protein expression level of PC (H, J), and relative PC activity (I, K) after ZFP36L2-AS overexpression or knockdown in vitro and in vivo. (L) The protein expression level of PC in ZFP36L2-AS overexpressed myoblast was analyzed after incubated with the protein synthesis inhibitor cycloheximide (CHX; 25 µg/ml). (M) The protein expression level of PC in ZFP36L2-AS knockdowned myoblast was analyzed after incubated with the proteasome inhibitor (MG-132; 5 µmol/L) for 12 h. N, O Relative acetyl-CoA content (N), and relative PC activity (O) with ACACA interference in vitro. P Relative acetyl-CoA content with ZFP36L2-AS overexpression or knockdown in vivo. Q, R Relative acetyl-CoA content (Q), and relative PC activity (R) induced by the listed nucleic acids. In panels (D, F, H, J, L, and M), the numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to β-Tubulin. Data are expressed as a fold-change relative to the control. Results are presented as mean ± SEM. In panels (E, G, I, K and N–P), statistical significance of differences between means was assessed using independent sample t-test. In panels (Q, R), ANOVA followed by Dunnett’s test was used. (*P < 0.05; **P < 0.01; N.S., no significant difference).
To further map the ZFP36L2-AS functional motifs corresponding to ACACA and PC binding, we conducted an in vitro RNA pull-down assay using a series of truncated ZFP36L2-AS fragments (Fig. 6C). This analysis revealed that nucleotides 816-1785 of ZFP36L2-AS are sufficient to interact with both ACACA and PC protein, while other ZFP36L2-AS truncated fragments could not (Fig. 6C). As the ZFP36L2-AS 816-1785 region is necessary for ZFP36L2-AS’s binding to ACACA and PC protein, we overexpressed the truncated fragment (816-1785 nt) and analyzed its impact on skeletal muscle development. Similar results to ZFP36L2-AS full-length overexpression were found (Supplementary Fig. 7A–F), implying that the interaction of ZFP36L2-AS with ACACA and PC protein may be a requisite for ZFP36L2-AS to function.
The function of ZFP36L2-AS partially depends on its regulation of ACACA and PC activity
With the observation that ZFP36L2-AS directly interacts with ACACA and PC, we further analyzed the effect of ZFP36L2-AS on ACACA and PC. Both in vitro and in vivo, the mRNA level of ACACA and PC were not changed with ZFP36L2-AS overexpression and knockdown (Supplementary Fig. 8A–D). Overexpression of ZFP36L2-AS significantly inhibited the phosphorylation level of ACACA protein and increased ACACA activity, whereas ZFP36L2-AS knockdown promoted ACACA phosphorylation and inactivated ACACA (Fig. 6D–G and Supplementary Fig. 7G, H), indicating that ZFP36L2-AS modulates the activity of ACACA by regulating ACACA phosphorylation. Next, we investigated the regulation of ZFP36L2-AS on the protein level and activity of PC. ZFP36L2-AS overexpression downregulated PC protein level and activity, while the protein level and activity of PC was increased with ZFP36L2-AS knockdown (Fig. 6H–K and Supplementary Fig. 7I, J). Cycloheximide (CHX) is a bacterial toxin that can inhibit protein biosynthesis. Treated with CHX decreased the expression of PC protein (Fig. 6L). However, ZFP36L2-AS knockdown relieved the decline of PC protein expression induced by CHX (Fig. 6L), suggesting that ZFP36L2-AS might promote the PC protein degradation. To further clarify the possible mechanism, the proteasome inhibitor MG-132 was used. MG-132 upregulated the protein levels of PC (Fig. 6M), suggesting that the inhibition of ubiquitin-proteasome pathway might ameliorate the degradation of PC. More importantly, MG-132 rescued the reduction of PC protein levels in ZFP36L2-AS-overexpressing myoblast (Fig. 6M). Overall, given that ZFP36L2-AS could modulate the ubiquitination level of total protein (Fig. 5K and Supplementary Fig. 5K), we infer ZFP36L2-AS induces the ubiquitination of PC to facilitate PC degradation, thereby inhibiting PC activity.
Acetyl-CoA is an allosteric activator of PC [38], has been found that can be catalyzed by ACACA to produce malonyl-CoA for fatty acid synthesis [39, 40], hinting that ACACA may affect the activity of PC by regulating acetyl-CoA. To verify this conjecture, ACACA was knockdown by specific siRNA (Supplementary Fig. 9A, B). As expected, ACACA knockdown increased acetyl-CoA content and enhanced the activity of PC (Fig. 6N, O). Similarly, ZFP36L2-AS knockdown augmented acetyl-CoA content, whereas the content of acetyl-CoA was reduced with overexpression of ZFP36L2-AS (Fig. 6P and Supplementary Fig. 7K). To further explore whether ZFP36L2-AS can reinforce the regulation of PC activity via ACACA, ZFP36L2-AS overexpression construct was co-transfected with ACACA specific siRNA. Partly, knockdown of ACACA abolished the downregulation of acetyl-CoA content and PC activity by ZFP36L2-AS overexpression (Fig. 6Q, R), demonstrated that ZFP36L2-AS can further enhance the inhibition of PC activity by activating ACACA.
Tissue expression profiles showed ACACA and PC highly expressed in breast and leg muscle (Supplementary Fig. 10A, D), implying that they may play an important role in skeletal muscle development. We further analyzed the expression of ACACA and PC during myoblast proliferation and differentiation. ACACA was slightly upregulated during myogenic differentiation, whereas the expression of PC was visibly decreased (Supplementary Fig. 10B, E). Moreover, subcellular location annotation showed that ACACA protein exists in nucleus and cytosol, while PC is localized in mitochondria (Supplementary Fig. 10C, F). To explore the potential biological functions of ACACA and PC in myogenesis, we performed a series of myoblast proliferation and differentiation assays. ACACA knockdown had similar results with PC overexpression, which promoted myoblast proliferation and inhibited myoblast differentiation (Supplementary Figs. 9C–L and 11A–L). In contrast, PC interference suppressed myoblast proliferation but facilitated myogenic differentiation (Supplementary Fig. 11M-X). Meanwhile, overexpression of PC promoted cellular mitochondrial respiration, whereas glycolytic capacity was increased with PC knockdown (Supplementary Fig. 12A-H).
Considering the expression and functional relationship between ZFP36L2-AS and ACACA and PC, we further determined the role of ACACA and PC on ZFP36L2-AS-mediated skeletal muscle development. Knockdown of ACACA improved fatty acid β-oxidation rate and upregulated the expression of CPT1, but downregulated key genes involved in fatty acid synthesis, which counteracts the inhibitory effect of ZFP36L2-AS on FAO (Fig. 7A, B). In addition, overexpression of PC rescued the suppression of cellular mitochondrial respiration induced by ZFP36L2-AS overexpression (Fig. 7C–F), as well as attenuates the activation of fast-twitch phenotype and muscle atrophy (Fig. 7G–J). Altogether, these results indicated that ACACA and PC are indispensable to the function of ZFP36L2-AS.
A, B) Relative fatty acid β-oxidation rate (A), and relative mRNA levels of fatty acid oxidation or synthesis related-genes (B) after co-transfection with the listed nucleic acids in CPMs. C–J OCR (C), basal respiration, maximal respiration and ATP production (D), ECAR (E), glycolysis and glycolytic capacity (F), relative mRNA expression levels of glycogenolytic and glycolytic genes (G), relative enzymes activity of LDH and SDH (H), relative mRNA expression levels of several fast-/slow-twitch myofiber genes (I), and relative mRNA expression of the atrophy and autophagy-related genes (J) induced by the listed nucleic acids in CPMs. Results are shown as mean ± SEM. In all panels, statistical significance of differences between means was assessed using ANOVA followed by Dunnett’s test. (*P < 0.05; **P < 0.01; N.S. no significant difference).
Discussion
Myogenesis is a highly ordered process including myoblast proliferation and differentiation, myotube formation and maturity, and is controlled by a series of myogenic regulatory factors [30, 41,42,43]. After birth, the number of myofibers in animals is basically fixed, and their skeletal muscle development is mainly regulated by the composition and size of myofibers. Recently, it is becoming increasingly clear that a complex network of epigenetic regulators and lncRNAs plays an essential role in skeletal muscle development [17,18,19,20, 44]. In our previous RNA-seq data, we identified lncRNA ZFP36L2-AS was highly expressed in fast growth rate broilers. Here, we found ZFP36L2-AS was upregulated during myogenic differentiation and highly expressed in breast and leg muscle, indicating its potential role in muscle development. Gain- and loss-of-function analysis revealed that ZFP36L2-AS inhibited myoblast proliferation but promoted myoblast differentiation in vitro. In vivo, ZFP36L2-AS activated fast-twitch muscle phenotype and induced muscle atrophy.
Skeletal muscle is a structurally and metabolically plastic tissue that maintains systemic energy homeostasis in response to various metabolic stresses [26]. Metabolic inflexibility in muscles is a dominant cause of various metabolic disorders [5]. Notably, recent evidences have revealed that the maintenance of skeletal muscle mass is closely related to muscle metabolism [8]. In this study, we found that ZFP36L2-AS repressed cellular mitochondrial respiration and fatty acid oxidation in skeletal muscle, resulting in excessive deposition of intramuscular fat. In the meantime, ZFP36L2-AS elevated glycolytic capacity and decrease oxidative capacity of skeletal muscle, which inactivated mTOR signaling, leading to the activation of UPS and autophagy-lysosomal system and induced muscle atrophy. Given that ZFP36L2-AS facilitated intramuscular fat deposition and induced muscle atrophy, ZFP36L2-AS could be a novel therapeutic target for obesity and sarcopenia.
Post-transcriptional regulation is an important form for lncRNA to regulate gene expression and function. Notably, increasing studies revealed that lncRNAs can be widely involved in a variety of biological processes through interacting with RBPs [44,45,46,47]. ACACA is a key enzyme in the process of fatty acid biosynthesis and oxidation, whose Ser79 site phosphorylation would inhibit the enzymatic activity of ACACA to promote FAO [48, 49]. In this study, we found ZFP36L2-AS can interact with ACACA through its 816-1785 region, thus inducing ACACA dephosphorylation and facilitating intramuscular fat deposition. In addition, PC (an enzyme that converts pyruvate to oxaloacetate), who has been reported to function as a RBP [45], was also discovered to bind with ZFP36L2-AS. ZFP36L2-AS damaged PC protein stability and inhibited PC activity, which may be attributed to the induction of ubiquitination by ZFP36L2-AS. Interestingly, we found ACACA can reduce the activity of PC by consuming acetyl-CoA, demonstrating that the inhibitory effect of ZFP36L2 on PC activity is partially ACACA-dependent.
Skeletal muscle is mainly composed of myofibers, which develop from myoblasts through a highly ordered biological process. In addition to myoblasts, skeletal muscle includes many muscle-resident cells such as blood cells, fibroblasts, preadipocytes, and satellite cells. Compared to other muscle-resident cells, we found that ZFP36L2-AS expression is more pronounced in myoblasts, blood cells and satellite cells. Since blood cells are mainly involved in oxygen transport and immune function of the body and ZFP36L2-AS regulates skeletal muscle development by mediating muscle metabolism, the function of ZFP36L2-AS in blood cells was not investigated in depth. On the other hand, inhibition and overexpression of ZFP36L2-AS did not change cellular ATP content in satellite cells, suggesting the expression of ZFP36L2-AS in satellite cells is not related to its role in muscle metabolism. Overall, the function of ZFP36L2-AS in muscle metabolism mainly depends on its expression in myoblasts, which contributes to its regulation of myogenesis and skeletal muscle development.
In summary, we identified lncRNA ZFP36L2-AS can interact with ACACA and PC to facilitate intramuscular fat deposition, as well as activate fast-twitch muscle phenotype and induce muscle atrophy (Supplementary Fig. 13). Our findings present a novel model about the regulation of lncRNA on muscle metabolism, and will contribute to the development of further research.
Materials and methods
Cell culture and transfection
Chicken primary myoblasts (CPMs) were isolated from the leg muscle of 11-day old chicken embryos and cultured as previously described [50]. To induce myogenic differentiation, growth medium was removed and replaced with differentiation medium (RPMI-1640 medium [Gibco, MD, USA] containing 2% horse serum after myoblasts achieving 90% cell confluence.
Fresh blood was collected from 14-day old chicken. After centrifugation at 1,500 × g, plasma was removed and blood cells were collected.
Chicken primary fibroblasts were isolated from the leg muscle of 10-day old chicken embryos. Fibroblasts were trypsinized and then collected by centrifugation as previously described [51]. Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Hyclone, USA) and 0.2% penicillin/streptomycin (Invitrogen, USA).
Chicken preadipocytes were isolated from 14-day old chicken as previously described [52], and cultured with DMEM/Ham’s nutrient mixture F-12 (DMEM/F12) basic medium with 10% (v/v) FBS (Hyclone, USA) and 0.2% penicillin/streptomycin (Invitrogen, USA).
Chicken satellite cells were isolated from leg muscle of 15-day old chicken embryos and cultured as previously described [53].
All cells were cultured at 37 °C in a 5% CO2 humidified atmosphere. And all transient transfections were performed using Lipofectamine 3000 Reagent (Invitrogen, USA) according to the manufacturer’s instructions.
RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (TaKaRa, Otsu, Japan) following the manufacturer’s protocol. Nuclear and cytoplasmic RNA fractionation was performed by using the PARIS Kit (Ambion, Life Technologies, USA) as recommended by the supplier. cDNA synthesis for mRNA was carried out using the PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Otsu, Japan). Real-time qPCR assay was performed as described before [54]. And primers used for RT-PCR and qRT-PCR are listed in Supplementary Table 4.
5ʹ and 3ʹ rapid-amplification of cDNA ends (RACE)
5′ and 3′ RACE of ZFP36L2-AS was performed using SMARTer RACE cDNA Amplification Kit (Clontech, Osaka, Japan) according to the manufacturer’s instructions. The gene-special primers used for RACE were presented in Supplementary Table 4.
Plasmids construction and RNA oligonucleotides
For Flag fusion protein construction, thirteen ORFs of ZFP36L2-AS were amplified and subcloned into HindIII and XhoI restriction sites in the pcDNA3.1-3xFlag-C vector.
For overexpression vectors construction, the full-length sequence and 816-1785 nt of ZFP36L2-AS and PC coding sequence (NCBI Reference Sequence: NM_204346.1) were amplified and cloned into the pcDNA-3.1 vector (Promega, Madison, WI, USA) by using the NheI and HindIII restriction sites.
For viral vectors constructed, the full-length sequence of ZFP36L2-AS was amplified, and then cloned into the adenoviral vector (pDC316-mCMV-ZsGreen; Addgene, Cambridge, MA, USA) between NheI and HindIII sites. Short hairpin RNA (shRNA) against ZFP36L2-AS was designed by Shanghai Hanbio Biotechnology Co., Ltd, and then subcloned into the pLVX-shRNA2-Puro vector (Addgene, Cambridge, MA, USA) by using the BamHI and EcoRI restriction sites.
The small interfering RNAs (siRNA) and antisense oligonucleotide (ASO) that were used for the specific knockdown of ZFP36L2-AS were designed and synthesized by Guangzhou RiboBio (Guangzhou, China). The siRNA against ACACA (NCBI Reference Sequence: NM_205505.1) and PC were also designed and synthesized.
The primers and oligonucleotide sequences used in this study are shown in Supplementary Tables 4 and 5.
Flow cytometry, 5-Ethynyl-2’-deoxyuridine (EdU) and cell counting kit-8 (CCK-8) assays
The experiments were performed as previously described [54]. In brief, the Cell Cycle Analysis Kit (Thermo Fisher Scientific, USA), C10310 EdU Apollo In Vitro Imaging Kit (RiboBio, China) and TransDetect Cell Counting Kit (TransGen, Beijing, China) were used for flow cytometry, EdU, and CCK-8 assay, as the manufacturer’s protocol.
Immunoblotting and immunofluorescence (IF)
Western blots were performed as previously described [50]. The primary antibodies used were anti-FLAG (AF519, 1:1,000, Beyotime), anti-ACACA (PA5-17564, 1:1000, Thermo Fisher Scientific), anti-p-ACACA Ser80 (orb315750, 1:500, Biorbyt), anti-PC (GTX132002, 1:500, GeneTex), anti-MYOD (ABP53067, 1:500, Abbkine), anti-MyHC (B103, 0.5 μg/ml, DHSB), anti-CPT1 (bs-23779R, 1:500, Bioss), anti-FASN (10624-2-AP, 1:200, Proteintech), anti-p-mTOR Ser2488 (#5536, 1:1000, CST), anti-mTOR (bs-1992R, 1:500; Bioss), Ubiquitin (#39361:1000, CST) anti-ULK1 (bs-3602R, 1:500; Bioss), anti-LC3B (NB100-2220, 2.0 ug/ml, Novus), anti-P62 (18420-1-AP, 1:1000, Proteintech) and anti-β-Tubulin (A01030, 1:10000, Abbkine). ProteinFind Goat Anti-Mouse IgG(H + L), HRP Conjugate (HS201-01, 1:1,000, TransGen) and ProteinFind Goat Anti-Rabbit IgG(H + L), HRP Conjugate (HS101-01, 1:500, TransGen) were used as a secondary antibody.
Immunofluorescence was performed using anti-MyHC (B103, 2.5 μg/ml, DHSB), as previously described [50]. A fluorescence microscope ((DMi8; Leica, German) was used to capture three randomly selected fields to visualize the area labeled with anti-MyHC.
Mitochondrial respiration assay
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of transfected myoblasts were measured using Seahorse XF Cell Mito Stress Test Kit and Seahorse XF Glycolysis Stress Test Kit (Agilent technologies, CA, USA) by a Seahorse XF96 Extracellular Flux Analyzer (Agilent technologies, CA, USA) following the manufacturer’s protocol, respectively.
Adenovirus/Lentivirus production and transduction
To generate adenovirus, the recombinant adentiviral expression plasmid was co-transfected with pHBAd-BHGlox ΔE1,3Cre plasmid using Lipofectamine 3000 reagent. After amplification, acquired adenovirus were purified with a ViraBind™ Adenovirus Purification Kit (Cell Biolabs, USA). Lentivirus production was performed as previously described [54]. Viral titers were evaluated by a gradient dilution.
1-day-old female chicks were randomly divided into two groups (Adv-ZFP36L2-AS and Adv-NC, or Lv-shZFP36L2-AS and Lv-shNC; n = 30), respectively. Chicks received two intramuscular doses of adenovirus (108 titers)/lentivirus (106 titers) in two different sites of the gastrocnemius. Thirteen days after the initial injection, chick gastrocnemius samples were collected from the above two groups.
Mitochondrial DNA (mtDNA) content and fatty acid oxidation (FAO) rate assay
Total DNA was extracted using the Tissue DNA Kit (D3396, Omega, GA, USA) according to the manufacturer’s instructions. The amount of mitochondrial DNA was determined by quantification of cytochrome c oxidase subunit II (COX2). The nuclear-encoded β-globin gene was used as internal controls. Primers used in this study can be found in the Supplementary Table 4.
The mitochondria of myoblast and gastrocnemius was isolated using the Cell/Tissue Mitochondria Isolation Kit (C3601/C3606, Beyotime, China). After measuring the mitochondrial protein concentration, freshly isolated mitochondria were subjected to FAO rate assay with the Colorimetric Fatty Acid Oxidation Rate Assay Kit (HL50679, Haling, Shanghai, China), according to the manufacturer’s protocol.
Central carbon metabolic profiling
ZFP36L2-AS knockdown gastrocnemius samples (n = 6) were used for metabolites extraction, and then performed on HPIC-MS/MS analysis. The high-performance ion exchange liquid chromatography (HPIC) separation was carried out using an Thermo Scientific Dionex ICS-6000 HPIC System (Thermo Fisher Scientific, IL, USA). An AB SCIEX 6500 QTRAP + triple quadrupole mass spectrometer (AB Sciex, USA), equipped with an electrospray ionization (ESI) interface, was applied for assay development.
Metabolic hierarchical clustering analysis (HCA) was performed using Cluster3.0 software as previously described [24].
Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC)
H&E staining was performed using the Hematoxylin and Eosin Staining Kit (Beyotime, Shanghai, China) following the manufacturer’s protocol. Immunohistochemistry was carried out using SP-POD Kit (SP0041, Solarbio, China) with primary antibodies included anti-MYH1(F59, 1:100, DHSB) and anti-MYH7 (S58, 1:300, DHSB).
Metabolite and enzyme activities assays
Content of adenosine triphosphate (ATP), triglyceride (TG), free fatty acid (FFA), glycogen, and acetyl-CoA as well as enzyme activity of lactic dehydrogenase (LDH), succinate dehydrogenase (SDH), acetyl-CoA carboxylase (ACC) and pyruvate carboxylase (PC) in skeletal muscle were measured using commercially available kits (BC0305, BC0625, BC0595, BC0345, BC0980, BC0685, BC0955, BC0410 and BC0730, Solarbio, China) according to the manufacturer’s instructions.
RNA pull-down assay
Ribo™ RNAmax-T7 biotin-labeled transcription kit (RiboBio, Guangzhou, China) was used to harvest biotinylated RNAs. RNA pull-down assays were performed with Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, IL, USA), according to the manufacturer’s instructions. The eluted products were identified by mass spectrometry with a Q Exactive mass spectrometer (Thermo fisher) or western blot. Differentially expressed genes (DEGs) were subjected to enrichment analysis of Gene Ontology (GO) functions and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
RNA immunoprecipitation (RIP) assay
RIP assays were performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, CA, USA) following the manufacturer’s protocol. The antibodies used for RIP assays were anti-ACACA (PA5-17564, 1:50, Thermo Fisher Scientific) and anti-PC (GTX132002, 1:100, GeneTex).
Statistical analysis
In this study, all experiments were repeated at least three times, and results were represented as mean ± SEM. Where applicable, the statistical significance of the data was tested using independent sample t-test or ANOVA followed by Dunnett’s test. The types of tests and the P values, when applicable, are indicated in the figure legends.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files). Additional data related to this paper may be available from the corresponding author on reasonable request.
References
Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–7.
Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol. 2016;78:85–107.
Ibrahim A, Neinast M, Arany ZP. Myobolites: muscle-derived metabolites with paracrine and systemic effects. Curr Opin Pharm. 2017;34:15–20.
Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25:1027–36.
Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–E1017.
Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13:460–6.
Buch A, Carmeli E, Boker LK, Marcus Y, Shefer G, Kis O, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age-An overview. Exp Gerontol. 2016;76:25–32.
Ferraro E, Pin F, Gorini S, Pontecorvo L, Ferri A, Mollace V, et al. Improvement of skeletal muscle performance in ageing by the metabolic modulator Trimetazidine. J Cachexia Sarcopenia Muscle. 2016;7:449–57.
Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci Stke. 2004;2004:re11.
Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183–90.
Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–9.
Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–27.
Wilusz JE, Freier SM, Spector DL. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–32.
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93.
Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4.
Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–23.
Wang S, Jin J, Xu Z, Zuo B. Functions and regulatory mechanisms of lncRNAs in skeletal myogenesis, muscle disease and meat production. Cells. 2019;8:1107.
Luo H, Lv W, Tong Q, Jin J, Xu Z, Zuo B. Functional non-coding RNA during embryonic myogenesis and postnatal muscle development and disease. Front Cell Dev Biol. 2021;9:628339.
Cai B, Ma M, Zhang J, Wang Z, Kong S, Zhou Z, et al. LncEDCH1 improves mitochondrial function to reduce muscle atrophy by interacting with SERCA2. Mol Ther Nucleic Acids. 2022;27:319–34.
Ma M, Cai B, Jiang L, Abdalla BA, Li Z, Nie Q, et al. lncRNA-Six1 is a target of miR-1611 that functions as a ceRNA to regulate Six1 protein expression and fiber type switching in chicken myogenesis. Cells. 2018;7:243.
Ouyang H, He X, Li G, Xu H, Jia X, Nie Q, et al. Deep sequencing analysis of miRNA expression in breast muscle of fast-growing and slow-growing broilers. Int J Mol Sci. 2015;16:16242–62.
Li Z, Cai B, Abdalla BA, Zhu X, Zheng M, Han P, et al. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J Cachexia Sarcopenia Muscle. 2019;10:391–410.
Lu Z, He X, Ma B, Zhang L, Li J, Jiang Y, et al. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J Agric Food Chem. 2017;65:11251–8.
Ding L, Yang X, Tian H, Liang J, Zhang F, Wang G, et al. Seipin regulates lipid homeostasis by ensuring calcium-dependent mitochondrial metabolism. EMBO J. 2018;37:e97572.
Li Q, Wang Y, Wu S, Zhou Z, Ding X, Shi R, et al. CircACC1 regulates assembly and activation of AMPK complex under metabolic stress. Cell Metab. 2019;30:157–73.
Yang X, Brobst D, Chan WS, Tse M, Herlea-Pana O, Ahuja P, et al. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci Signal. 2019;12:eaau1468.
Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37.
Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531.
Koutakis P, Weiss DJ, Miserlis D, Shostrom VK, Papoutsi E, Ha DM, et al. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol. 2014;2:921–8.
Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011;12:349–61.
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14.
Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci USA. 2011;108:8674–9.
Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–54.
Lin Z, Zhang B, Liu X, Jin R, Zhu W. Effects of chicory inulin on serum metabolites of uric acid, lipids, glucose, and abdominal fat deposition in quails induced by purine-rich diets. J Med Food. 2014;17:1214–21.
Cappel DA, Deja S, Duarte J, Kucejova B, Inigo M, Fletcher JA, et al. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019;29:1291–305.
Adina-Zada A, Zeczycki TN, Attwood PV. Regulation of the structure and activity of pyruvate carboxylase by acetyl CoA. Arch Biochem Biophys. 2012;519:118–30.
Abu-Elheiga L, Jayakumar A, Baldini A, Chirala SS, Wakil SJ. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci USA. 1995;92:4011–5.
Cronan JJ, Waldrop GL. Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res. 2002;41:407–35.
Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525–32.
Buckingham M, Rigby PW. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28:225–38.
Saccone V, Puri PL. Epigenetic regulation of skeletal myogenesis. Organogenesis. 2010;6:48–53.
Jin JJ, Lv W, Xia P, Xu ZY, Zheng AD, Wang XJ, et al. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc Natl Acad Sci USA. 2018;115:E9802–E9811.
Ma MZ, Zhang Y, Weng MZ, Wang SH, Hu Y, Hou ZY, et al. Long noncoding RNA GCASPC, a target of miR-17-3p, negatively regulates pyruvate carboxylase-dependent cell proliferation in gallbladder cancer. Cancer Res. 2016;76:5361–71.
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705–15.
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang Y, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33:706–20.
Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269:22162–8.
Castle JC, Hara Y, Raymond CK, Garrett-Engele P, Ohwaki K, Kan Z, et al. ACC2 is expressed at high levels in human white adipose and has an isoform with a novel N-terminus [corrected]. PLoS One. 2009;4:e4369.
Cai B, Ma M, Chen B, Li Z, Abdalla BA, Nie Q, et al. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018;9:367.
Schmid C, Steiner T, Froesch ER. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983;161:117–21.
Zhang T, Zhang X, Han K, Zhang G, Wang J, Xie K, et al. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS One. 2017;12:e0172389.
Bai C, Hou L, Li F, He X, Zhang M, Guan W. Isolation and biological characteristics of beijing Fatty chicken skeletal muscle satellite cells. Cell Commun Adhes. 2012;19:69–77.
Cai B, Li Z, Ma M, Zhang J, Kong S, Abdalla BA, et al. Long noncoding RNA SMUL suppresses SMURF2 production-mediated muscle atrophy via nonsense-mediated mRNA decay. Mol Ther Nucleic Acids. 2021;23:512–26.
Funding
This work was supported by the Natural Scientific Foundation of China (U1901206, 31802051 and 31761143014), Local Innovative and Research Teams Project of Guangdong Province (2019BT02N630), National Key R&D Program of China (2021YFD1300100), China Agriculture Research System (CARS-41-G03), Guangdong Basic and Applied Basic Research Foundation (2021A1515111069), and the Science and Technology Program of Guangdong province, China (2020B1212060060).
Author information
Authors and Affiliations
Contributions
QN and XZ conceived and designed the study. BC and MM performed the experiments, interpreted the data and wrote the paper. JZ, SK, and ZZ performed the experiments. ZL, BAA, HX, and RAL interpreted the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal experimental protocols were conformed to “The Instructive Notions with Respect to Caring for Laboratory Animals” issued by the Ministry of Science and Technology of the People’s Republic of China, and approved by the Institutional Animal Care and Use Committee at the South China Agricultural University (approval ID: 2020-C010).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cai, B., Ma, M., Zhang, J. et al. Long noncoding RNA ZFP36L2-AS functions as a metabolic modulator to regulate muscle development. Cell Death Dis 13, 389 (2022). https://doi.org/10.1038/s41419-022-04772-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-04772-2
This article is cited by
-
MYH1G-AS is a chromatin-associated lncRNA that regulates skeletal muscle development in chicken
Cellular & Molecular Biology Letters (2024)