Abstract
Injury to the axons of retinal ganglion cells (RGCs) is a key pathological event in glaucomatous neurodegeneration. The transcription factors JUN (the target of the c-Jun N-terminal kinases, JNKs) and DDIT3/CHOP (a mediator of the endoplasmic reticulum stress response) have been shown to control the majority of proapoptotic signaling after mechanical axonal injury in RGCs and in other models of neurodegeneration. The downstream transcriptional networks controlled by JUN and DDIT3, which are critical for RGC death, however, are not well defined. To determine these networks, RNA was isolated from the retinas of wild-type mice and mice deficient in Jun, Ddit3, and both Jun and Ddit3 three days after mechanical optic nerve crush injury (CONC). RNA-sequencing data analysis was performed and immunohistochemistry was used to validate potential transcriptional signaling changes after axonal injury. This study identified downstream transcriptional changes after injury including both neuronal survival and proinflammatory signaling that were attenuated to differing degrees by loss of Ddit3, Jun, and Ddit3/Jun. These data suggest proinflammatory signaling in the retina might be secondary to activation of pro-death pathways in RGCs after acute axonal injury. These results determine the downstream transcriptional networks important for apoptotic signaling which may be important for ordering and staging the pro-degenerative signals after mechanical axonal injury.
Similar content being viewed by others
Introduction
Axonal injury is a critical component of many neurodegenerative diseases including glaucoma. Multiple studies show an early insult occurs to retinal ganglion cell (RGC) axons at the optic nerve head (ONH) in glaucoma that triggers signaling pathways that result in axonal degeneration and somal death [1,2,3,4,5,6,7,8]. Specifically, JUN, the canonical target of the JNKs, and CHOP/DDIT3, an important mediator of the PERK arm of endoplasmic reticulum (ER) stress signaling, are key mediators of somal apoptosis after axonal injury in RGCs. Individually, JUN and DDIT3 play important roles in apoptotic RGC death after axonal injuries including mechanical optic nerve injury and ocular hypertensive injury [9,10,11,12,13,14,15,16]. Dual deficiency of JUN and DDIT3 prevented the majority of RGC death for extended time periods after optic nerve crush (CONC)—120 days after CONC, Ddit3/Jun deficient retinas had ~74% improved RGC survival compared to wild-type controls [17]. In this study, Junf alleles were only recombined from ~80% of RGCs [17], suggesting these two transcription factors control nearly all proapoptotic signaling in RGCs after mechanical axonal injury. Since JUN and DDIT3’s canonical roles are as transcription factors, it is likely JUN and DDIT3 control the transcriptional nodes required for somal apoptosis after axonal injury. To date, only limited transcriptional targets of JUN and DDIT3 (i.e., ATF3, BIM) have been identified that have also been shown to be important for somal death after axonal injury. Importantly, none of these targets have been shown to phenocopy the protection to RGCs afforded by JUN and DDIT3 deficiency [10, 11, 18, 19].
Glaucomatous neurodegeneration is complex, with activation of multiple signaling pathways that may have both pro-survival and pro-degenerative roles after injury [7, 20,21,22,23]. Due to this complexity, the specific molecular cascade from inciting injury to axonal degeneration and somal apoptosis remains largely undefined. As JUN and DDIT3 are transcription factors that control the majority of proapoptotic signaling, determining the transcriptional targets of these molecules will likely identify the activation of key pro-death molecules.
Given the profound protection to RGCs after mechanical axonal injury in mice deficient in Jun and Ddit3, it is clear that these molecules are key transcriptional nodes governing axonal injury-induced RGC death. Thus, understanding the transcriptional response controlled by these genes after axonal injury will provide a detailed understanding of the molecular events that control RGC death after axonal injury. To define the transcriptional network(s) controlling RGC death after axonal injury (CONC), RNA sequencing of whole retinas was performed from wild-type mice and mice deficient in Ddit3, Jun or both Jun and Ddit3. Our results showed both neuronal and immune transcriptional networks are activated after axonal injury and that activation of these networks was differentially altered by loss of Ddit3, Jun, or Ddit3/Jun.
Materials and Methods
Mice
All experiments were approved by the University of Rochester’s Committee on Animal Resources and conducted in adherences with the Association for Research in Vision and Ophthalmology statement on the use of animals in ophthalmic and vision research. Animals received chow and water ad libitum and were housed on a 12-h light-dark cycle. Three alleles were used to generate four strains for these experiments as has been previously described [17]. Briefly, these strains include 1) germline deficient Ddit3 mice (Jackson Laboratory, Bar Harbor, ME, Stock 005530) [referred to as Ddit3−/−], 2) a floxed allele of Jun [24] recombined with the Six3cre, a neural retina cre [25] [referred to as Six3cre+; Junf/f or Jun−/−], 3) mice deficient in both Jun and Ddit3 [referred to as Six3cre+; Junf/f; Ddit3−/− or Jun−/−Ddit3−/−] and 4) wild-type control mice, such as Six3cre+; Jun+/+, Six3cre-; Junf/f, Six3cre−; Jun+/f, Six3cre−; Jun+/+, and Ddit3+/+ [collectively referred to as WT]. B6.Gt(ROSA)26Sortm75.1(CAG-tdTomato*)Hze/J (TdTomato+; Jackson Laboratory, Bar Harbor, ME, Stock 025106) mice were used to assess AAV2.2-cmv-gfp-cre recombination efficiency. All mice were backcrossed at least five generations to C57BL/6 J mice. The number of animals of each genotype and treatment (determined by power analysis) was stated in Additional file 11. Experimenters were masked to genotype and condition. Mice of each genotype group were randomly selected for experimental analysis.
Controlled optic nerve crush
Mice of 2–6 months of age were used for controlled optic nerve crush (CONC) after anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. The optic nerve was surgically exposed and crushed just behind the globe for five seconds with self-closing forceps (Roboz RS-5027, Gaithersburg, MD) [8, 18]. Ophthalmic ointment containing neomycin, polymyxin b sulfates, and dexamethasone was applied to both eyes following the procedure (Sandoz, Princeton, NJ). Control eyes included those that underwent sham surgery (optic nerve exposed but not crushed) and those eyes that were not manipulated except receiving antibiotic ointment (naïve eyes) as optic nerve crush is known to lead to microglial alterations in the contralateral eye [26, 27]. Eyes were then harvested for RNA extraction and immunohistochemistry.
NMDA Intravitreal injections
NMDA intravitreal injections were performed as previously described [28], Briefly, animals were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. The sclera was cleared with the bevel of a 33 G needle, which was then used to poke a small hole just behind the limbus. A Hamilton needle was used to deliver 2 μL of 100 mM NMDA (Sigma-Aldrich, M3262) or PBS (vehicle control). Injections were performed over the course of approximately 2 minutes to avoid sudden increases in intraocular pressure.
RGC-specific deletion of Jun f alleles with adenoassociated viral cre delivery
To generate retinas with RGC-specific deletion of Jun, Junf/fDdit3−/− animals were bilaterally intravitreally injected with 1 μL of stock AAV2.2-cmv-gfp-cre (UNC vector core). To generate WT controls, 1 μL of AAV2.2-Cmv-cre-Gfp or AAV2.2-Cmv-Gfp (with no cre allele, UNC vector core) was intravitreally injected into the eyes of Jun+/+Ddit3+/+ animals. CONC was performed no earlier than 28 days after viral delivery to ensure sufficient recombination and endogenous protein degradation. To test recombination robustness and specificity, AAV2.2-Cmv-cre-Gfp was intravitreally injected into the eyes of Tdtomato+ animals, and Tdtomato expression was quantified for specific cell types.
RNA sample preparation and sequencing
Dissections were completed in RNase-free conditions. Retinas were quickly dissected in cold PBS and then submerged in RNA-later (Qiagen 76106, Germantown, MD). Retinas were kept at room temperature for 24 h and then stored in RNA-later at −80 °C. RNA extraction and sequencing were performed by The Jackson Laboratory Genome Technologies Core. Retina tissues were homogenized with TRIzol (Invitrogen, Carlsbad, CA) as previously described [29]. RNA was isolated and purified using the QIAGEN miRNeasy mini extraction kit in accordance with manufacturer’s instructions. RNA quality was measured via the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) and poly(A) RNA-seq sequencing libraries were compiled by TruSeq RNA Sample preparation kit v2 (Illumina, San Diego, CA). Quantification was performed using qPCR (Kapa Biosystems, Wilmington, MA). RNA-seq was performed on the Illumina HiSeq4000 sequencer for 75 bp pair-end reads according to the manufacturer’s instructions.
RNA-sequencing data analysis
RNA-sequencing data analysis were performed as previously described [29]. Briefly, fastq files of all samples were subjected to the removal of adapters and trimming low-quality bases (Phred < 30) using NGSQCToolkit v2.3 [30]. RSEM v1.2.12 was used to quantify gene expression using the trimmed reads as input [31]. RSEM internally utilizes Bowtie2 v2.2.0 as its aligner [32] with supplied annotations at default parameters against the C57BL/6 J mouse genome (mm10). Genes that had less than 1 count per million (cpm) in at least two samples were removed before normalization. Differentially expressed (DE) gene analysis was performed using Quasi-Likelihood methods in edgeR 3.20.9 package [33]. DE genes were determined comparing CONC with naïve eye group within each genotype (treatment effect) and between genotypes (interaction of treatment and genotype effect). DE genes were uploaded onto Ingenuity Pathway Analysis (IPA) software for canonical pathway, upstream regulator, disease and function, and regulatory effect analysis. Please see statistical analysis section for criteria of DE genes and downstream analyses in IPA.
Immunohistochemistry
All eyes for immunohistochemistry were fixed in 4% paraformaldehyde (PFA) for 2 hours and then stored in 1 M phosphate-buffered saline (PBS) as has been previously described [9, 17]. For flat-mount staining, the anterior segment was removed and then the retina was carefully dissected from the optic cup. After PBS washes, retinas were blocked with 10% horse serum in 0.4% TritonX in PBS at 4 degrees and then incubated in primary antibodies (Additional file 12) for three overnights at 4 °C before one overnight in secondary antibodies (Alexafluor-conjugated, Invitrogen) also at 4 °C. Retinas were then washed and mounted (RGC side up) in Flurogel (Electron Microscopy Sciences, Hatfield, PA). For cryosections, the anterior segment was removed and the posterior segment was processed. After PBS washes, 14 µm cryosections were blocked with 10% horse serum in 0.1% TritonX in PBS at room temperature for several hours and then incubated in primary antibodies (Additional file 12) overnight at 4 °C. The next day cryosections were incubated with secondary antibodies (Alexafluor-conjugated, Invitrogen & JacksonImmuno, West Grove, PA) for several hours at room temperature and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI, ThermoFisher, Waltham, MA) before mounting with Flurogel.
Statistical analysis
A minimum of four retinas (both sexes mixed) were used for each experimental cohort and condition (Additional file 11). Significant DE genes resulting from treatment or treatment by genotype effect were defined with a false discovery rate (FDR) less than 0.05 [i.e. −log10(FDR) > 1.3] and an absolute fold change (FC) larger than 1.0. Significantly enriched canonical pathways were determined by a Benjamini-Hochberg (B-H) p value less than 0.05 [i.e. -log10(B-H p-value)>1.3]. Significantly enriched upstream regulators were defined by a Fisher exact p value less than 0.05 [i.e. −log10(p value) > 1.3]. Activation of a pathway or upstream regulator was determined by z-score equal or more than 2 and vice versa.
Results
Jun, Ddit3, and dual Jun/Ddit3 deficiency differentially alters retinal transcriptional profiles in response to axonal injury
To characterize transcriptional changes that occur after axonal injury, whole WT, Jun−/−, Ddit3−/−, and Jun/Ddit3−/− retinas were dissected for RNA sequencing three days after controlled optic nerve crush (CONC), a time point at which RGC death has just begun [18]. Principle component analysis (PCA) demonstrated the overall changes in the transcriptome between CONC and naïve conditions (DNT, did not touch) and across individual genotypes (Additional file 1a). The experimental samples were primarily separated by treatment effect (CONC vs. naïve, 9.54% variance explained) and then further modestly separated by genotype effect (WT, Ddit3−/−, Jun−/− and Jun−/−Ddit3−/−, 8% variance explained). Differentially expressed (DE) genes were determined by comparing CONC with naïve retinas for all four experimental groups (WT, Jun−/−, Ddit3−/−, Jun/Ddit3−/−) using edgeR (see Methods). As expected, Jun and Ddit3 were significantly elevated in WT retinas after CONC (Additional file 1b). Further, Jun was elevated in Ddit3−/− retinas and Ddit3 was elevated in Jun−/− retinas. This was consistent with previous reports that demonstrated Jun deletion did not prevent DDIT3 expression in RGCs [10], and Ddit3 deletion did not prevent JUN activation in RGCs [17], suggesting these genes did not regulate each other after optic nerve injury.
Overall, compared to naïve eyes, 1264 DE genes were detected in WT animals after CONC with a false discovery rate (FDR) of less than 0.05 (Fig. 1a). Retinas deficient in Ddit3, Jun, or Jun/ Ddit3 had 744, 383, and 286 DE genes respectively in response to CONC. Some CONC-induced DE genes were genotype-specific while others overlapped across genotypes (Fig. 1b). Increased RGC survival correlated with fewer DE genes. Previous reports have shown deficiency in Ddit3, Jun, and Jun/Ddit3 increased RGC survival after CONC with Ddit3 deficiency having the least effect and Jun/Ddit3 deficiency having the greatest effect (35 days post-CONC; WT: 21.6%, Ddit3: 47.5%, Jun: 75.4%, Jun/Ddit3: 88.5%) [17]. Thus, the number of DE genes resulting from injury in each genotype cohort correlated with RGC survival.
CONC led to differential expression of genes in wildtype (WT), Ddit3 (Ddit3−/−), Jun (Jun−/−), and dual Jun/Ddit3 (Jun−/−Ddit3−/−) deficient retinas (a). Venn diagram depicting the overlap of CONC-responding DE genes in all experimental groups (b). Expression of inflammatory signaling genes including complement genes and Gfap in WT, Jun, Ddit3, and dual Jun/Ddit3 deficient retinas after CONC as compared to unmanipulated retinas (c). Area marked with a dot indicates the fold change of a gene is not significant (FDR ≥ 0.05) in a given genotype group. Area without a dot indicates the fold change of a gene is significant (FDR < 0.05) in a given genotype group. To validate the gene expression data, retinal sections were stained for C1QA and counterstained with DAPI to assess complement signaling after axonal injury. C1QA immunofluorescence was increased after axonal injury in the retinal nerve fiber layer and inner plexiform layer in WT animals after CONC injury as compared to sham retinas, however, these changes were not observed in Jun, Ddit3, or dual Jun/Ddit3 deficient retinas after CONC (d N = 3 per genotype; Scale bar: 50μm). Gfap expression was also increased in WT animals but not Jun deficient or dual Jun/Ddit3 deficient retinas after CONC as compared to unmanipulated retinas (c). Retinal sections were stained for GFAP and counterstained with DAPI after axonal injury. GFAP + presumed Müller glial processes were evident after axonal injury in WT animals as compared to sham retinas, however, these changes were not observed in dual Jun/Ddit3 deficient retinas after CONC (e N = 3 per genotype; Scale bar: 50μm).
Canonical pathway analysis (Ingenuity Pathway Analysis, IPA) was employed to determine biological processes enriched in DE genes across genotypes. Analyses predicted multiple canonical pathways were modulated by CONC in WT retinas but not in retinas deficient in Jun, Ddit3 and/or Jun/Ddit3 (Additional file 1C, Additional file 3). These included numerous RGC-intrinsic injury signaling pathways such as ‘neuronal signaling’, ‘extrinsic inflammatory signaling’ and ‘cholesterol biosynthesis’. Compared to WT retinas, fewer pathways were significantly enriched in response to CONC in Ddit3, Jun, and dual Jun/Ddit3 deficient animals with the dual Jun/Ddit3 deficient animals having the fewest significantly enriched pathways. Jun deficiency appeared to lessen the response to axonal injury to a larger degree than Ddit3 deficiency. It was also intriguing that apparent RGC-extrinsic proinflammatory canonical pathways were increased after axonal injury, however, these same signals were not activated in Ddit3, Jun, and dual Jun/Ddit3 deficient retinas. These results indicate a multitude of transcriptional changes occur prior to the onset of cell death in RGCs and likely also in retinal glial cells. For instance, canonical pathway analyses predicted an attenuation of the complement cascade that has been shown to be important in neurodegeneration and to be an early extrinsic component of glaucomatous neurodegeneration [34,35,36,37,38,39]. Components of the complement cascade, including the initiating factors of the classical pathway C1qa, C1qb, and C1qc, were activated in WT retinas after CONC but were largely suppressed by Ddit3, Jun, and dual Jun/Ddit3 deficiency (Fig. 1c). Additional glial genes such as Itgam (microglia) and Gfap (astrocytes, Müller glia) showed a similar pattern of expression to complement genes (Fig. 1c). To validate these findings, immunofluorescence was performed on retinal sections seven days after CONC for C1QA and GFAP. After CONC, C1Q + immunoreactivity was increased in the innermost layer of the retina near the inner limiting membrane, presumably labeling astrocytes or Müller glia endfeet as has been described in previous studies in WT retinas [36, 40], as compared to uninjured WT animals (Fig. 1d). This increase in C1Q+ immunofluorescence was markedly less in retinas from Ddit3- Jun-, and dual Jun/Ddit3-deficient mice. Similarly, Ddit3-, Jun-, and dual Jun/Ddit3-deficient retinas had less GFAP + immunoreactivity compared to WT after CONC. Immunoreactivity was primarily limited to the nerve fiber layer (NFL) and ganglion cell layer (GCL) in WT retinal sections, presumably labeling retinal astrocytes in addition to possible Müller glia endfeet (Fig. 1e). These data suggest extrinsic inflammatory responses to axonal injury in glial cells is dependent upon or mediated by intrinsic RGC signaling.
Jun, Ddit3, and dual Jun/Ddit3 specific signaling after axonal injury
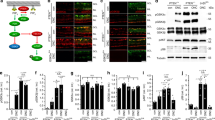
In order to determine specific Jun, Ddit3, and dual Jun/Ddit3 targets in response to axonal injury, DE genes that were affected by the interaction of CONC treatment and gene type were determined using edgeR (FDR < 0.05, Fig. 2a). A total of 73 CONC-responding genes were predicted to be ‘regulated’ by Jun. Of these, 23 were unique to Jun, with 50 shared with Ddit3 (47 genes) or Jun/Ddit3 (3 genes, Fig. 2a). Enrichment of the 73 genes revealed 35 genes were related to cell death and survival including neuronal cell death, apoptosis of neurons, and survival of ganglion cells (Fig. 2b, Table 1, Additional file 5). Genes predicted to be activated by Jun included Ecel1 (endothelin converting enzyme-like 1), Lif (leukemia inhibitory factor), Jak3 (janus kinase 3), and known Jun targets Atf3 and Hrk [10] (Fig. 2c,d). Ecel1 is a member of the M13 family of endopeptidases that are important regulators of neuropeptide and peptide hormone signaling. ECEL1 (also known as DINE), has previously been shown to be activated in response to injury in both the peripheral nervous system and central nervous system– with its activation dependent upon genes including Jun, Atf3, and Lif [41]. Genes predicted to be inhibited by Jun included Ccer2 (Coiled-Coil Glutamate Rich Protein 2), Pou4f2 (POU Class 4 Homeobox 2), and Isl2 (SL LIM Homeobox 1, Fig. 2e, f). Only 4 CONC-responding genes were predicted to be regulated by Ddit3, with three of these also regulated by Jun (Fig. 3a). These included Avil, Gm12889, Stk32a, and Stbd1 (Fig. 3b, c). Interestingly, 93 CONC-responding genes were predicted to be regulated by Jun and Ddit3 (dual Jun/Ddit3). Of the 93, 43 were predicted to be regulated by a combination of both Jun and Ddit3 (Fig. 4a). These included Cdsn, Myc, Col16a1, and Bbc3 that were activated and Hydin, Npy1r, Clstn2, and Ano3 that were inhibited by Jun/Ddit3 (Fig. 4b, c). Collectively, this analysis reveals putative novel targets of Jun and Ddit3 in axonal injury for future testing.
The DE genes resulting from interaction of CONC treatment and genotype were determined using the following equation in edgeR package. The category of the DE genes included the CONC-responding genes dependent on Jun (J.CONC genes), dependent on Ddit3 (D.CONC genes), or dependent on both Jun and Ddit3 (JD.CONC). J.CONC genes = (Jun−/− CONC – Jun−/− DNT) – (WT CONC – WT DNT), D.CONC genes = (Ddit3−/− CONC – Ddit3−/− DNT) – (WT CONC – WT DNT), JD.CONC genes = (Jun−/−Ddit3−/− CONC – Jun−/− Ddit3−/− DNT) - (WT CONC – WT DNT). The Venn diagram shows the overlap of the DE genes as a result of interaction of CONC treatment and genotype, highlighting the CONC-responding genes affected by Jun (a). Cell death and survival was determined to be the top molecular and cellular function as determined by IPA analysis (shown in Table 1). The top disease and function annotations of the cell death and survival category were found to be neuronal cell death, apoptosis of neurons, and survival of ganglion cells (b). The fold change of top 10 CONC-responding genes activated (c) and inhibited (e) by Jun in all genotypes. Area marked with a dot indicates the fold change of a gene is not significant (FDR ≥ 0.05) in a given genotype group. Area without a dot indicates the fold change of a gene is significant (FDR < 0.05) in a given genotype group. Box plots demonstrate the expression levels of activated (d) and inhibited (f) genes in all genotypes after CONC (orange) as compared to unmanipulated retinas (DNT, green).
The Venn diagram shows the overlap of the DE genes as a result of interaction of CONC treatment and genotype, highlighting the CONC-responding genes affected by Ddit3 (a). The fold change of CONC-responding genes altered by Ddit3 in all genotypes (b). Area marked with a dot indicates the fold change of a gene is not significant (FDR ≥ 0.05) in a given genotype group. Area without a dot indicates the fold change of a gene is significant (FDR < 0.05) in a given genotype group. Box plots demonstrate the expression levels of CONC-responding genes affected by Ddit3 in all genotypes after CONC (orange) as compared to unmanipulated retinas (c, DNT, green).
The Venn diagram shows the overlap of the DE genes as a result of interaction of CONC treatment and genotype, highlighting the CONC-responding genes affected by Jun and Ddit3 together (a). The fold change of top 10 CONC-responding genes activated (b) and inhibited (d) by Jun and Ddit3 together in all genotypes. Area marked with a dot indicates the fold change of a gene is not significant (FDR ≥ 0.05) in a given genotype group. Area without a dot indicates the fold change of a gene is significant (FDR < 0.05) in a given genotype group. Box plots demonstrate the expression levels of activated (c) and inhibited (e) genes in all genotypes after CONC (orange) as compared to unmanipulated retinas (DNT, green).
Ddit3, Jun, and dual Jun/Ddit3 deficiency attenuate glial responses after axonal injury
IPA upstream regulator analysis was then performed to determine transcriptional hubs downstream of JUN and DDIT3 (Additional file 6a, Fig. 5). This analysis identified potentially key differences between samples deficient in Jun and/or Ddit3. For example, the upstream regulator ATF4 was largely activated in response to CONC in WT mice (Additional file 6b) but BDNF was suppressed in WT mice after injury (Additional file 6c). Regulatory effect analysis predicted a group of regulators (CAMP, GAPDH, NCSTN, CCN5) controlled a network of genes involved in activation and migration of myeloid cells and phagocytes, only in WT but not in Jun and/or Ddit3 deficient groups (Table 2, Fig. 5a). Myeloid and microglia proinflammatory signaling has been thought to contribute to pathogenic signaling in neurodegenerative conditions, including after mechanical optic nerve injury [42,43,44,45,46,47,48]. This network contained 19 upregulated genes comparing CONC to DNT in WT retinas that were differentially suppressed by Jun, Ddit3, or dual Jun/Ddit3 deficiency. For instance, Itgb2, C3ar1, Csf1, and Stat1 were suppressed by Jun deficiency; Cd44 and Ctss were suppressed by Ddit3 deficiency; and Csf1r and Jun were suppressed by dual Jun/Ddit3 deficiency (Fig. 5b, c).
The IPA regulator effect revealed the top upstream regulator network leading to myeloid cell-related functions in WT mice (a red or blue color indicates the trend of activation or inhibition of an upstream regulator, target gene, or their predicted function, respectively). The top 5 upstream regulator networks among all genotypes were summarized in Table 1. The fold change of the target genes in the top regulator effect was reported for all genotypes (b). Genes were grouped by the significance level of the fold change observed in each strain (Group 1 gene: not affected by Jun, Ddit3, and Jun/Ddit3 together; Group II gene: modulated by Jun, Ddit3, or Jun/Ddit3 together; Group III: genes modulated by only Jun, Group IV: genes affected by either Jun or Ddit3 but not Jun/Ddit3 together; group V: genes modulated by Jun/Ddit3 together). Area marked with a dot indicates the fold change of a gene is not significant (FDR ≥ 0.05) in a given genotype group. Area without a dot indicates the fold change of a gene is significant (FDR < 0.05) in a given genotype group. Box plots demonstrate the expression levels of selected gene related to myeloid cells functions in all genotypes after CONC (orange) as compared to unmanipulated retinas (c DNT, green).
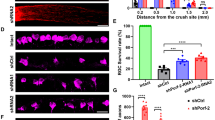
Only three genes (Itga5, Spp1, Tac1) remained activated in all genotypes, suggesting the majority of neuroinflammatory activity after axonal injury is mediated through Jun and/or Ddit3, but that some degree of microglial response is independent of Jun and/or Ddit3. To test this, microglia activation was evaluated using IBA1 (a marker of microglia/macrophages) and CD68 (a marker of phagocytic microglia). IBA1+ cells were assessed in whole-mount retinas from all experimental (CONC) and control (SHAM) cohorts seven days after CONC (Fig. 6a). Compared to WT sham retinas, IBA1+ immunofluorescence was greatly increased in WT retinas after CONC at both the ONH and peripheral retina. IBA1+ ‘streaks’ were apparent in WT animals after CONC that appeared to follow the path of some RGC axons as they extend from the optic nerve. In contrast to the ramified IBA1+ cells in sham retinas, amoeboid morphology was apparent after CONC in WT animals. IBA1+ immunofluorescence was noticeably less in Ddit3-, Jun- and Jun/Ddit3-deficient retinas compared to WT after CONC. Jun and Jun/Ddit3 deficient retinas displayed a largely ramified morphology after injury, while Ddit3 deficient animals displayed amoeboid morphology after injury. CD68 was also differentially expressed in all experimental cohorts as compared to sham control retinas of the same genotype (Fig. 5c). As compared with a paucity of CD68+ cells in sham retinas, multiple CD68+ cells were observed after CONC in the inner retina in WT retinas, with the majority observed in the GCL and IPL. CD68+ immunoreactivity was comparatively decreased in Ddit3, Jun and dual Jun/Ddit3 deficient retinas (Fig. 6b). Together, these findings support previous reports that retinal glial activation occurs early after axonal injury and confirm transcriptional data that these proinflammatory responses are attenuated by Jun and Ddit3 deficiency. Also, the level of microglial activation inversely correlated to RGC survival previously observed in each of these cohorts at extended timepoints after CONC [17].
Whole-mount retinas were stained with IBA1 to label microglia/macrophages 7 days after CONC. As compared to WT sham retinas, IBA + immunofluorescence was greatly increased in WT retinas after CONC at both the optic nerve head (ONH) and peripheral retina. IBA + immunofluorescence was decreased in Ddit3 deficient animals and greatly diminished in Jun and dual Jun/Ddit3 deficient retinas (a N ≥ 5 per genotype. Scale bars: 50 μm) Retinal sections were also stained for CD68 and counterstained with DAPI 7 days after CONC. Increased CD68 + immunofluorescence was evident after CONC in WT retinas as compared to sham retinas, however, these changes were not observed in the dual Jun/Ddit3 deficient retinas after CONC (b, N = 3 per genotype; Scale bar: 50μm).
Given Jun/Ddit3 deletion prevented the majority of RGC apoptosis after CONC injury [17], it is likely CONC-induced glial responses are triggered by RGC-intrinsic JUN/DDIT3 activation and subsequent apoptosis. However, JUN accumulation in macroglia has been observed after glaucoma-relevant injury [49, 50] and has been implicated in driving glial activation [51, 52]. Six3cre has been shown to recombine floxed alleles in retinal macroglia in addition to retinal neurons [17, 25, 53]. Thus, it remained possible Jun/Ddit3 deletion from glial cells resulted in attenuated glial responses after CONC.
To determine whether glial activation is triggered by RGC-intrinsic JUN/DDIT3 activation and subsequent apoptosis, or alternatively as a result of glia-intrinsic JUN/DDIT3 activation, WT and Jun−/−Ddit3−/− animals were subjected to excitotoxic N-methyl D-aspartic acid (NMDA) injury. Unlike after CONC injury [17], Jun/Ddit3 deletion did not prevent RGC death after NMDA injury [28]. Thus, this experimental paradigm allowed the assessment of Jun/Ddit3-deficient glia in response to RGC death. NMDA caused a robust increase in expression of C1Q, IBA1, CD68, and GFAP in Müller glia processes. These changes were not attenuated by glial Jun/Ddit3 deletion (Additional file 8a-d). Likewise, increased ONH immunofluorescence of IBA1 and CD68 was not attenuated by Jun/Ddit3 deletion from glia (Additional file 8e-g). These data suggest Jun/Ddit3-deficient glia are capable of activation in response to RGC death, thus suggesting attenuation of glial activation after CONC was not necessarily due to loss of glial JUN/DDIT3.
To further investigate the importance of RGC-intrinsic JUN/DDIT3 activation in mediating CONC-induced glial activation, Junf alleles were specifically recombined from RGCs using a virally delivered Cmvcre recombinase. As assessed with a TdTomato reporter line, AAV2.2-Cmv-cre-Gfp robustly recombined floxed alleles in RGCs, and only recombined floxed alleles in a small subset of SOX2 + Müller glia, GFAP + astrocytes, and IBA1 + microglia, and did not recombine floxed alleles in the ONH (Additional file 9). RGC-specific Jun deletion prevented CONC-induced increases in expression of C1Q, GFAP in Müller glia processes, IBA1, and CD68 (Additional file 10). Taken together, these data strongly implicate the importance of RGC-intrinsic JUN/DDIT3 signaling and subsequent apoptosis in driving glial activation after CONC.
Discussion
Axonal injury is an important component of the pathogenesis of glaucoma and other neurodegenerative diseases. Unfortunately, the critical molecular signaling pathways leading from axonal injury to glaucomatous neurodegeneration are not well defined. Deficiency in the transcription factors Jun and Ddit3 provides robust, long-term protection of RGC somas after axonal injury. Defining the transcriptional response controlled by these genes after axonal injury will lead to a deeper understanding of the molecular events that control axonal injury-induced RGC death, which will likely be necessary for the development of treatments for optic neuropathies [7]. However, to date, few transcriptional targets of Jun and Ddit3 have been identified. To identify the downstream transcriptional targets of these molecules, whole retina RNA sequencing was performed after acute axonal injury (CONC). This approach allowed for the study of both intrinsic and extrinsic transcriptional signaling networks in retinas where intrinsic pro-death signaling had been arrested.
JUN and DDIT3 mediated intrinsic signaling in RGCs after axonal injury
After axonal injury in the optic nerve, degeneration of the RGC soma and axon is known to be compartmentalized—that is, distinct molecular signaling pathways control axonal degeneration and somal apoptosis [1, 7, 8, 54, 55]. Transcriptional changes in the soma are also known to contribute to axonal degeneration programs after injury, including those driven by retrograde JNK-JUN signaling [56, 57].
Our study revealed Ddit3 and Jun mediate activation of overlapping and distinct RGC-intrinsic processes after axonal injury. Several of these genes have been previously identified, including Bim, Hrk, and Atf3. Interestingly, deficiency of these genes did not phenocopy Jun and/or Ddit3 deficiency after axonal injury [10, 18]. Transcriptional changes related to cell metabolism were also identified in these data, and metabolic changes are thought to play an important early role in RGC neurodegeneration [58,59,60,61]. Specific genes identified in these data that are known to be important for cellular metabolism include Nmnat2, Nad, Sqstm1, Slc3a2, and Slc7a11. Unfortunately, the transcriptional changes mediated by Ddit3 and Jun that could be critical for controlling RGC death after axonal injury were not readily apparent in this dataset. Numerous recently published studies have assessed transcriptional changes in RGCs at the single-cell level in unmanipulated retinas and after axonal injury [62, 63]. Using bioinformatic tools to compare the data sets generated here with studies like Tran et al. [62] will be a powerful approach to unravel the critical transcriptional changes that control RGC death after axonal injury in glaucoma and perhaps other optic neuropathies.
Jun and Ddit3 deficiency uncovers extrinsic events not necessarily correlated with RGC loss
Retinal neurodegeneration after the axonal injury is complex because multiple cell types and multiple signaling pathways contribute to RGC death. Jun/Ddit3 deficiency provides sustained, robust protection to RGCs after axonal injury, and thus these data allowed for the study of the transcriptional changes that occur in other retinal cell types in the absence of significant RGC death. Ddit3, Jun, and Jun/Ddit3 deficiency prevented most of the neuroinflammatory signaling that occurs after CONC. For instance, both Ddit3 and Jun appear to be upstream of complement activation in the retina after CONC (Figs. 1–2). This was supported by immunofluorescence showing reduced levels of C1Q in Jun/Ddit3 deficient mice (Fig. 1). In addition, Jun/Ddit3 deficiency appeared to lessen the presence of activated and phagocytic microglia (Fig. 6). Neuroinflammatory processes have been suggested as early extrinsic drivers of neurodegeneration in the retina [42, 48, 64, 65], but these data predict the earliest signaling after the axonal injury is intrinsic to RGCs. In accordance with this hypothesis, RGC-specific deletion of Jun prevented increases in C1Q, GFAP, IBA1, and CD68 immunofluorescence after CONC (Additional file 10). Thus, our data suggest RGC-intrinsic JUN activity, rather than glial JUN activity, accounts for changes in inflammatory markers after CONC. However, some immune related-genes and pathways were differentially expressed in all genotypes after optic nerve crush. These included the myeloid-related transcription factor Spp1, a gene which we have shown is important in neuroinflammatory responses [29]. These data show some extrinsic processes could not be prevented by deletion of Jun/Ddit3, highlighting the need for further studies exploring the relationship between RGC pro-death factors and extrinsic neuroinflammatory processes. Future work should consider single-cell sequencing of glaucoma-relevant cell types to improve the resolution of the molecular control of RGC death.
Limitations of this study and future directions
A limitation to our experimental approach is the Ddit3 allele is a germline deletion, while the Jun allele is a floxed allele, which was recombined with the inner neural retinal cre Six3cre. Six3cre is known to recombine alleles in astrocytes and likely Müller glia, as it is expressed in early retinal progenitor cells [25, 53]. Thus, it is possible the RGC protection afforded by this model of Jun and Ddit3 deficiency and the transcriptional changes that occur in each experimental cohort after CONC may be confounded by the manipulation of signaling pathways in retinal cells other than RGCs. One example of this possibility is that intravitreal injection of TNF activates JUN in Müller glia. Intravitreal injection of TNF prior to CONC was protective of RGCs, and the mechanism for this protection has been proposed to be JUN activity in Müller glia [66]. Given retinal injury may activate JUN in other cell types, which might serve protective or deleterious roles, it is important to interpret whole retina transcriptional changes within these limitations. Another factor that might confound the analysis of transcriptional data is the recombination efficiency of Six3cre (known to recombine Jun floxed alleles in ~80% of RGCs) [9, 17]. Given this, it is possible the number of significantly regulated genes in Jun and Ddit3/Jun deficient retinas may represent some genes which are strongly expressed in cells that continue to express JUN. Thus, the number of differentially regulated genes may in fact be lower than reported in Jun and Ddit3/Jun deficient retinas. Finally, we have previously shown an uncoupling of the transcriptional response in the retina and ONH in glaucoma. In this study, we did not profile the ONH where changes to supporting cells, such as astrocytes, microglia, and vascular cells, such as endothelial cells and pericytes may be similarly important in determining axonal degeneration or survival.
Conclusions
Transcriptional changes after axonal injury are an important part of the signaling cascade driving RGC death. We identified the transcriptional changes that occur downstream of Jun and Ddit3, two transcription factors that are known to control the majority of axonal injury-induced RGC death. By specifically identifying the transcriptional changes that occur in retinas where intrinsic pro-death signaling has been arrested, these data also allowed for the study of RGC extrinsic signaling changes that occur after axonal injury. We determined neuronal and proinflammatory signaling is significantly upregulated after axonal injury; Ddit3, Jun, and Jun/Ddit3 deficiency attenuate differentially expressed genes after injury; and finally, proinflammatory changes after acute axonal injury in the retina are likely secondary to RGC apoptosis rather than a primary driver of retinal neurodegeneration. These results identify downstream networks of Jun and Ddit3 that may be broadly important for neurodegeneration.
Data availability
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)). The sequencing dataset discussed in this publication is deposited at NCBI’s Gene Expression Omnibus (GEO accession number GSE168789).
References
Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–37.
Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66.
Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–25.
Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:986–96.
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49.
Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–83.
Syc-Mazurek SB, Libby RT. Axon injury signaling and compartmentalized injury response in glaucoma. Prog Retin Eye Res. 2019;73:100769
Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26.
Syc-Mazurek SB, Fernandes KA, Libby RT. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis. 2017;8:e2945.
Fernandes KA, Harder JM, Kim J, Libby RT. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp Eye Res. 2013;112:106–17.
Fernandes KA, Harder JM, Fornarola LB, Freeman RS, Clark AF, Pang IH, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis. 2012;46:393–401.
Hu Y, Park KK, Yang L, Wei X, Yang Q, Cho KS, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–52.
Yang L, Li S, Miao L, Huang H, Liang F, Teng X, et al. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. J Neurosci. 2016;36:5891–903.
Isenmann S, Bahr M. Expression of c-Jun protein in degenerating retinal ganglion cells after optic nerve lesion in the rat. Exp Neurol. 1997;147:28–36.
Kwong JM, Caprioli J. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Exp Eye Res. 2006;82:576–82.
Levkovitch-Verbin H, Quigley HA, Martin KR, Harizman N, Valenta DF, Pease ME, et al. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp Eye Res. 2005;80:663–70.
Syc-Mazurek SB, Fernandes KA, Wilson MP, Shrager P, Libby RT. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Mol Neurodegeneration. 2017;12:71.
Harder JM, Fernandes KA, Libby RT. The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep. 2012;2:530.
Jauhiainen A, Thomsen C, Strombom L, Grundevik P, Andersson C, Danielsson A, et al. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PloS One. 2012;7:e33208.
Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retinal Eye Res. 2012;31:702–19.
Maes ME, Schlamp CL, Nickells RW. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog Retinal Eye Res. 2017;57:1–25.
Howell GR, Soto I, Libby RT, John SW. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2013;246:54–61.
Farley MM, Watkins TA. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu Rev Pathol. 2018;13:93–116.
Behrens A, Sibilia M, David JP, Mohle-Steinlein U, Tronche F, Schutz G, et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002;21:1782–90.
Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–2.
Panagis L, Thanos S, Fischer D, Dermon CR. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci. 2005;21:2305–9.
Ramirez AI, Salazar JJ, de Hoz R, Rojas B, Gallego BI, Salobrar-Garcia E, et al. Macro- and microglial responses in the fellow eyes contralateral to glaucomatous eyes. Prog Brain Res. 2015;220:155–72.
Fahrenthold BK, Fernandes KA, Libby RT. Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death. Sci Rep. 2018;8:4641.
Yang H, Graham LC, Reagan AM, Grabowska WA, Schott WH, Howell GR. Transcriptome profiling of brain myeloid cells revealed activation of Itgal, Trem1, and Spp1 in western diet-induced obesity. J Neuroinflammation. 2019;16:169.
Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One. 2012;7:e30619.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma. 2011;12:323.
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Lun AT, Chen Y, Smyth GK. It’s DE-licious: A Recipe for Differential Expression Analyses of RNA-seq Experiments Using Quasi-Likelihood Methods in edgeR. Methods Mol Biol. 2016;1418:391–416.
Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, et al. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 2014;71:44–52.
Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Investig. 2011;121:1429–44.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, et al. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010;51:5071–82.
Bonifati DM, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010.
Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380.
Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47:1024–9.
Kiryu-Seo S, Kato R, Ogawa T, Nakagomi S, Nagata K, Kiyama H. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J Biol Chem. 2008;283:6988–96.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7.
Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investigative Ophthalmol Vis Sci. 2008;49:1437–46.
Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519:599–620.
Nadal-Nicolas FM, Jimenez-Lopez M, Salinas-Navarro M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J Neuroinflammation. 2017;14:218.
Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–65.
Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. 2014;4:a017269.
Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Investig. 2012;122:1246–61.
Hashimoto K, Parker A, Malone P, Gabelt BT, Rasmussen C, Kaufman PS, et al. Long-term activation of c-Fos and c-Jun in optic nerve head astrocytes in experimental ocular hypertension in monkeys and after exposure to elevated pressure in vitro. Brain Res. 2005;1054:103–15.
Chidlow G, Wood JPM, Casson RJ. Investigations into Hypoxia and Oxidative Stress at the Optic Nerve Head in a Rat Model of Glaucoma. Front Neurosci. 2017;11:478.
MacDonald JM, Doherty J, Hackett R, Freeman MR. The c-Jun kinase signaling cascade promotes glial engulfment activity through activation of draper and phagocytic function. Cell Death Differ. 2013;20:1140–8.
Albanito L, Reddy CE, Musti AM. c-Jun is essential for the induction of Il-1β gene expression in in vitro activated Bergmann glial cells. Glia. 2011;59:1879–90.
Rattner A, Yu H, Williams J, Smallwood PM, Nathans J. Endothelin-2 signaling in the neural retina promotes the endothelial tip cell state and inhibits angiogenesis. Proc Natl Acad Sci USA. 2013;110:E3830–3839.
Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–71.
Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retinal Eye Res. 2005;24:639–62.
Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, et al. Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell. 2016;164:1031–45.
Maor-Nof M, Romi E, Sar Shalom H, Ulisse V, Raanan C, Nof A, et al. Axonal Degeneration Is Regulated by a Transcriptional Program that Coordinates Expression of Pro- and Anti-degenerative Factors. Neuron. 2016;92:991–1006.
Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–60.
Inman DM, Harun-Or-Rashid M. Metabolic Vulnerability in the Neurodegenerative Disease Glaucoma. Front Neurosci. 2017;11:146.
Leruez S, Marill A, Bresson T, de Saint Martin G, Buisset A, Muller J, et al. A Metabolomics Profiling of Glaucoma Points to Mitochondrial Dysfunction, Senescence, and Polyamines Deficiency. Invest Ophthalmol Vis Sci. 2018;59:4355–61.
Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010;30:5644–52.
Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron. 2019;104:1039–55 e1012.
Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018;9:2759.
Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40.
Chen H, Cho KS, Vu THK, Shen CH, Kaur M, Chen G, et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9:3209.
Mac Nair CE, Fernandes KA, Schlamp CL, Libby RT, Nickells RW. Tumor necrosis factor alpha has an early protective effect on retinal ganglion cells after optic nerve crush. J Neuroinflammation. 2014;11:194.
Acknowledgements
The authors would like to acknowledge Dr. Jeffery Harder for helpful discussions about the manuscript and Dr. Yasuhide Furuta (Six3-cre) and and Drs. Erwin Wagner and Jason Stoller (Junf) for generously providing mouse strains. The authors would also like to thank the Genomic Technology at the Jackson Laboratory for RNA extraction and RNA-sequencing service. This work was supported by EY018606 (RTL), EY027701 (GRH, RTL), EY030739 (OJM), Research to Prevent Blindness (an unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center), and the Diana Davis Foundation Chair for Glaucoma Research (GRH). The funding agencies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors designed and interpreted the experiments. All authors both edited the manuscript and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Nicolas Bazan
Supplementary information
41419_2022_4666_MOESM7_ESM.xlsx
Complete list of the top Diseases and Functions Annotation enriched from J.CONC gene under the Cell Death and Survival category
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syc-Mazurek, S.B., Yang, H.S., Marola, O.J. et al. Transcriptional control of retinal ganglion cell death after axonal injury. Cell Death Dis 13, 244 (2022). https://doi.org/10.1038/s41419-022-04666-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-04666-3