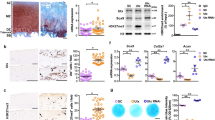
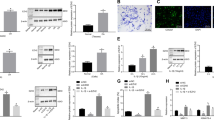
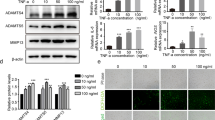
Abstract
Osteoarthritis (OA) is one of the most common joint diseases, there are no effective disease-modifying drugs, and the pathological mechanisms of OA need further investigation. Here, we show that H3K36 methylations were decreased in senescent chondrocytes and age-related osteoarthritic cartilage. Prrx1-Cre inducible H3.3K36M transgenic mice showed articular cartilage destruction and osteophyte formation. Conditional knockout Nsd1Prrx1-Cre mice, but not Nsd2Prrx1-Cre or Setd2Prrx1-Cre mice, replicated the phenotype of K36M/+; Prrx1-Cre mice. Immunostaining results showed decreased anabolic and increased catabolic activities in Nsd1Prrx1-Cre mice, along with decreased chondrogenic differentiation. Transcriptome and ChIP-seq data revealed that Osr2 was a key factor affected by Nsd1. Intra-articular delivery of Osr2 adenovirus effectively improved the homeostasis of articular cartilage in Nsd1Prrx1-Cre mice. In human osteoarthritic cartilages, both mRNA and protein levels of NSD1 and OSR2 were decreased. Our results indicate that NSD1-induced H3K36 methylations and OSR2 expression play important roles in articular cartilage homeostasis and OA. Targeting H3K36 methylation and OSR2 would be a novel strategy for OA treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary information files). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020;396:1711–2.
Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384:51–9.
Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early-stage symptomatic osteoarthritis of the knee - time for action. Nat Rev Rheumatol. 2021;17:621–32.
Simkin PA. A biography of the chondrocyte. Ann Rheum Dis. 2008;67:1064–8.
Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic science of articular cartilage. Clin Sports Med. 2017;36:413–25.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200.
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13:115–26.
Xu L, Zhao Z, Dong A, Soubigou-Taconnat L, Renou JP, Steinmetz A, et al. Di- and Tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in arabidopsis thaliana. Mol Cell Biol. 2023;28:1348–60.
Fang D, Gan H, Lee JH, Han J, Wang Z, Riester SM, et al. The histone H3.3K36M mutation reprograms the epigenome ofchondroblastomas. Science. 2016;352:1344–8.
Lu C, Jain SU, Hoelper D, Bechet D, Molden RC, Ran L, et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science. 2016;352:844–9.
Rayasam GV, Wendling O, Angrand PO, Mark M, Niederreither K, Song L, et al. NSD1 is essential for early post‐implantation development and has a catalytically. EMBO J. 2003;22:3153–63.
Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet. 2002;30:365–6.
Baujat G, Rio M, Rossignol S, Sanlaville D, Lyonnet S, Le Merrer M, et al. Paradoxical NSD1 Mutations in Beckwith-Wiedemann Syndrome and 11p15 Anomalies in Sotos Syndrome. Am J Hum Genet. 2004;74:715–20.
Agwu JC, Shaw NJ, Kirk J, Chapman S, Ravine D, Cole TRP. Growth in Sotos syndrome. Arch Dis Child. 1999;80:339–42.
Visser R, Landman EB, Goeman J, Wit JM, Karperien M. Sotos syndrome is associated with deregulation of the MAPK/ERK-signaling pathway. PLoS One. 2012;7:e49229.
Shao R, Zhang Z, Xu Z, Ouyang H, Wang L, Ouyang H, et al. H3K36 methyltransferase NSD1 regulates chondrocyte differentiation for skeletal development and fracture repair. Bone Res. 2021;9:30.
Haseeb A, Kc R, Angelozzi M, de Charleroy C, Rux D, Tower RJ, et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci USA. 2021;118:e2019152118.
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–81.
Fafian-Labora JA, Rodriguez-Navarro JA, O’Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab. 2020;32:71–86.e5.
Zhuang L, Jang Y, Park YK, Lee JE, Jain S, Froimchuk E, et al. Depletion of Nsd2-mediated histone H3K36 methylation impairs adipose tissue development and function. Nat Commun. 2018;9:1796.
Lian WS, Wu RW, Ko JY, Chen YS, Wang SY, Yu CP, et al. Histone H3K27 demethylase UTX compromises articular chondrocyte anabolism and aggravates osteoarthritic degeneration. Cell Death Dis. 2022;13:538.
Wang C, Shen J, Ying J, Xiao D, O’Keefe RJ. FoxO1 is a crucial mediator of TGF-beta/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc Natl Acad Sci USA. 2020;117:30488–97.
Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–28.
Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604.
Lam PY, Kamei CN, Mangos S, Mudumana S, Liu Y, Drummond IA. odd‐skipped related 2 is required for fin chondrogenesis in zebrafish. Dev Dyn. 2013;242:1284–92.
Gao Y, Lan Y, Liu H, Jiang R. The zinc finger transcription factors Osr1 and Osr2 control synovial joint formation. Dev Biol. 2011;352:83–91.
Chen X, Gong W, Shao X, Shi T, Zhang L, Dong J, et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis. 2022;81:87–99.
Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80.
Nohno T, Koyama E, Myokai F, Taniguchi S, Ohuchi H, Saito T, et al. Development biology-A chicken homeobox gene related to drosophila paired is predominantly expressed in the developing limb. Dev Biol. 1993;158:254–64.
Bragdon BC, Bennie A, Molinelli A, Liu Y, Gerstenfeld LC. Post natal expression of Prx1 labels appendicular restricted progenitor cell populations of multiple tissues. J Cell Physiol. 2022;237:2550–60.
Zanoni P, Steindl K, Sengupta D, Joset P, Bahr A, Sticht H, et al. Loss-of-function and missense variants in NSD2 cause decreased methylation activity and are associated with a distinct developmental phenotype. Genet Med. 2021;23:1474–83.
Boczek NJ, Lahner CA, Nguyen TM, Ferber MJ, Hasadsri L, Thorland EC, et al. Developmental delay and failure to thrive associated with a loss‐of‐function variant in WHSC1 (NSD2). Am J Med Genet Part A. 2018;176:2798–802.
Edmunds JW, Mahadevan LC, Clayton AL. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 2007;27:406–20.
Zhang Y, Fang Y, Tang Y, Han S, Jia J, Wan X, et al. SMYD5 catalyzes histone H3 lysine 36 trimethylation at promoters. Nat Commun. 2022;13:3190.
Wang L, Niu N, Li L, Shao R, Ouyang H, Zou W. H3K36 trimethylation mediated by SETD2 regulates the fate of bone marrow mesenchymal stem cells. PLoS Biol. 2018;16:e2006522.
Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 2009;323:1232–4.
Su Z, Zong Z, Deng J, Huang J, Liu G, Wei B, et al. Lipid metabolism in cartilage development, degeneration, and regeneration. Nutrients. 2022;14:3984.
Villalvilla A, Gómez R, Largo R, Herrero-Beaumont G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int J Mol Sci. 2013;14:20793–808.
Zhu S, Makosa D, Miller B, Griffin TM. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect Tissue Res. 2019;61:34–47.
Yoshimoto M, Sadamori K, Tokumura K, Tanaka Y, Fukasawa K, Hinoi E. Bioinformatic analysis reveals potential relationship between chondrocyte senescence and protein glycosylation in osteoarthritis pathogenesis. Front Endocrinol. 2023;14:1153689.
Murphy CL, Thoms BL, Vaghjiani RJ, Lafont JE. HIF-mediated articular chondrocyte function: prospects for cartilage repair. Arthritis Res Ther. 2009;11:213.
Arden NK, Perry TA, Bannuru RR, Bruyere O, Cooper C, Haugen IK, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17:59–66.
Sabha M, Hochberg MC. Non-surgical management of hip and knee osteoarthritis; comparison of ACR/AF and OARSI 2019 and VA/DoD 2020 guidelines. Osteoarthritis and Cartilage Open. 4 (2022).
Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2018;15:77–90.
Acknowledgements
We thank Dr. Xinyuan Liu (Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai) for providing vectors and reagents related to the adenovirus package. We also thank the members of the Zou lab for helpful discussions.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFA1106800, 2022YFA0806600), the National Natural Science Foundation of China (NSFC) (82230082, 81991512, 82202742, 81902212), the Strategic Priority Research Program of the Chinese Academy of Science (XDB0570000), the CAS Project for Young Scientists in Basic Research (YSBR-077), the Shanghai Frontiers Science Center of Degeneration and Regeneration in Skeletal System (BJ1- 9000- 22- 4002).
Author information
Authors and Affiliations
Contributions
WZ and RS designed the research; RS, JS, ZZ performed the research; LZ, KG, CZ and QB contributed reagents and analytical tools; MK and QB collected human cartilage samples; RS, JS, ZZ and WZ analyzed the data; RS and WZ wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experimental protocol for animal studies was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the CAS Center for Excellence in Molecular Cell Sciences, Chinese Academy of Sciences. Human articular cartilage samples were obtained with the informed consent of the patients and the approval of the ethics committee of Shanghai Sixth People’s Hospital and Zhejiang Provincial People’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, R., Suo, J., Zhang, Z. et al. H3K36 methyltransferase NSD1 protects against osteoarthritis through regulating chondrocyte differentiation and cartilage homeostasis. Cell Death Differ 31, 106–118 (2024). https://doi.org/10.1038/s41418-023-01244-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01244-8
This article is cited by
-
Unlocking the potential of β-1,3-xylooligosaccharides from Caulerpa lentillifera: structural characterization, antioxidative and anti-osteoarthritis applications
Chemical and Biological Technologies in Agriculture (2024)