Abstract
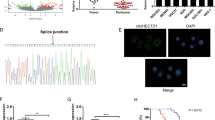
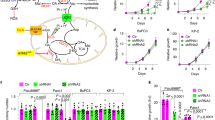
Mitochondrial malic enzyme 2 (ME2), which catalyzes the conversion of malate to pyruvate, is frequently upregulated during tumorigenesis and is a potential target for cancer therapy. However, the regulatory mechanism underlying ME2 activity is largely unknown. In this study, we demonstrate that ME2 is highly expressed in human colorectal cancer (CRC) tissues, and that ME2 knockdown inhibits the proliferation of CRC cells. Furthermore, we reveal that ME2 is succinylated and identify Sirtuins 5 (SIRT5) as an ME2 desuccinylase. Glutamine deprivation directly enhances the interaction of SIRT5 with ME2 and thus promotes SIRT5-mediated desuccinylation of ME2 at lysine 346, activating ME2 enzymatic activity. Activated ME2 significantly enhances mitochondrial respiration, thereby counteracting the effects of glutamine deprivation and supporting cell proliferation and tumorigenesis. Additionally, the levels of succinylated ME2 at K346 and SIRT5 in CRC tissues, which are negatively correlated, are associated with patient prognosis. These observations suggest that SIRT5-catalyzed ME2 desuccinylation is a key signaling event through which cancer cells maintain mitochondrial respiration and promote CRC progression under glutamine deficiency conditions, offering the possibility of targeting SIRT5-mediated ME2 desuccinylation for CRC treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The full and uncropped western blots were uploaded as the Supplemental Material. Other datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.
References
Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34:355–77.
Xu D, Shao F, Bian X, Meng Y, Liang T, Lu Z. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 2021;33:33–50.
Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–24.
Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, et al. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell. 2015;57:537–51.
Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15.
Li Y, Bie J, Zhao L, Song C, Zhang T, Li M, et al. SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis. Cell Death Differ. 2023;30:1916–30.
Sedlak JC, Yilmaz OH, Roper J. Metabolism and colorectal cancer. Annu Rev Pathol. 2023;18:467–92.
Sainero-Alcolado L, Liano-Pons J, Ruiz-Perez MV, Arsenian-Henriksson M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022;29:1304–17.
Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20:745–54.
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31.
Wei Z, Song J, Wang G, Cui X, Zheng J, Tang Y, et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun. 2018;9:4468.
Qiao S, Lu W, Glorieux C, Li J, Zeng P, Meng N, et al. Wild-type IDH2 protects nuclear DNA from oxidative damage and is a potential therapeutic target in colorectal cancer. Oncogene. 2021;40:5880–92.
Liu L, Qi L, Knifley T, Piecoro DW, Rychahou P, Liu J, et al. S100A4 alters metabolism and promotes invasion of lung cancer cells by up-regulating mitochondrial complex I protein NDUFS2. J Biol Chem. 2019;294:7516–27.
Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–93.
Wang YP, Sharda A, Xu SN, van Gastel N, Man CH, Choi U, et al. Malic enzyme 2 connects the Krebs cycle intermediate fumarate to mitochondrial biogenesis. Cell Metab. 2021;33:1027–1041.e8.
Zhao M, Yao P, Mao Y, Wu J, Wang W, Geng C, et al. Malic enzyme 2 maintains protein stability of mutant p53 through 2-hydroxyglutarate. Nat Metab. 2022;4:225–38.
Dey P, Baddour J, Muller F, Wu CC, Wang H, Liao WT, et al. Genomic deletion of malic enzyme 2 confers collateral lethality in pancreatic cancer. Nature. 2017;542:119–23.
Yang Y, Zhang Z, Li W, Si Y, Li L, Du W. alphaKG-driven RNA polymerase II transcription of cyclin D1 licenses malic enzyme 2 to promote cell-cycle progression. Cell Rep. 2023;42:112770.
Yang Y, Gibson GE. Succinylation Links Metabolism to Protein Functions. Neurochem Res. 2019;44:2346–59.
Yang X, Wang Z, Li X, Liu B, Liu M, Liu L, et al. SHMT2 desuccinylation by SIRT5 drives cancer cell proliferation. Cancer Res. 2018;78:372–86.
Tong Y, Guo D, Lin SH, Liang J, Yang D, Ma C, et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell. 2021;81:2303–16 e8.
Li W, Kou J, Zhang Z, Li H, Li L, Du W. Cellular redox homeostasis maintained by malic enzyme 2 is essential for MYC-driven T cell lymphomagenesis. Proc Natl Acad Sci USA. 2023;120:e2217869120.
Wang YQ, Wang HL, Xu J, Tan J, Fu LN, Wang JL, et al. Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner. Nat Commun. 2018;9:545.
Hao Y, Samuels Y, Li Q, Krokowski D, Guan BJ, Wang C, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun. 2016;7:11971.
Verdin E, Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16:258–64.
Hsieh JY, Chen KC, Wang CH, Liu GY, Ye JA, Chou YT, et al. Suppression of the human malic enzyme 2 modifies energy metabolism and inhibits cellular respiration. Commun Biol. 2023;6:548.
Zhu Y, Gu L, Lin X, Liu C, Lu B, Cui K, et al. Dynamic regulation of ME1 Phosphorylation and Acetylation Affects Lipid Metabolism and Colorectal Tumorigenesis. Mol Cell. 2020;77:138–49.e5.
Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–24.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Edwards DN, Ngwa VM, Raybuck AL, Wang S, Hwang Y, Kim LC, et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J Clin Invest. 2021;131:e140100.
Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166:555–66.
Pei X, Li KY, Shen Y, Li JT, Lei MZ, Fang CY, et al. Palmitoylation of MDH2 by ZDHHC18 activates mitochondrial respiration and accelerates ovarian cancer growth. Sci China Life Sci. 2022;65:2017–30.
Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang Y, et al. Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol Cell. 2017;65:917–931.e6.
Wang X, Shi X, Lu H, Zhang C, Li X, Zhang T, et al. Succinylation inhibits the enzymatic hydrolysis of the extracellular matrix protein fibrillin 1 and promotes gastric cancer progression. Adv Sci. 2022;9:e2200546.
Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. 2013;441:191–5.
Qi H, Ning X, Yu C, Ji X, Jin Y, McNutt MA, et al. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 2019;10:170.
Wang K, Hu Z, Zhang C, Yang L, Feng L, Yang P, et al. SIRT5 contributes to colorectal cancer growth by regulating T cell activity. J Immunol Res. 2020;2020:3792409.
Liu X, Rong F, Tang J, Zhu C, Chen X, Jia S, et al. Repression of p53 function by SIRT5-mediated desuccinylation at Lysine 120 in response to DNA damage. Cell Death Differ. 2022;29:722–36.
Hu T, Shukla SK, Vernucci E, He C, Wang D, King RJ, et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology. 2021;161:1584–600.
Sarfraz I, Rasul A, Hussain G, Hussain SM, Ahmad M, Nageen B, et al. Malic enzyme 2 as a potential therapeutic drug target for cancer. IUBMB Life. 2018;70:1076–83.
Acknowledgements
We thank Professor Li Jia (Shanghai Institute of Materia Medica, Chinese Academy of Sciences) for providing the pRH-ME2 plasmids. We also thank Professor Yu Wei (Fudan University) for providing the SIRT5-HA and SIRT5-HA H158Y plasmids. This work was supported by the Natural Science Foundation of China (82002964, 82173063), China Postdoctoral Science Foundation (2022M711370), Wuxi Taihu Lake Talent Plan for Leading Talents in Medical and Health Profession, Wuxi Medical Key Discipline (ZDXK2021002) and Youth Program of Wuxi Medical Foundation (Q202123).
Author information
Authors and Affiliations
Contributions
CL, PT, and ZH designed the research. CL and PT performed most of the experiments. KC performed bioinformatic analyses. SY and BF collected clinical samples. ZH and CL supervised the project. CL, PT, FL, and ZH wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Clinical Research Ethics Committees of Jiangnan University (JN. No20220515b0320730[151]).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teng, P., Cui, K., Yao, S. et al. SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration. Cell Death Differ 31, 65–77 (2024). https://doi.org/10.1038/s41418-023-01240-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01240-y