Abstract
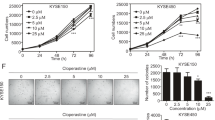
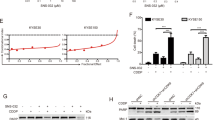
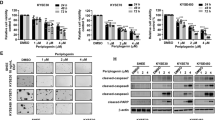
Esophageal squamous cell carcinoma (ESCC) is one of the most common human malignancies worldwide and is associated with high morbidity and mortality. Current treatment options are limited, highlighting the need for development of novel effective agents. Here, a high-throughput drug screening (HTS) was performed using ESCC cell lines in both two- and three-dimensional culture systems to screen compounds that have anti-ESCC activity. Our screen identified romidepsin, a histone deactylase inhibitor, as a potential anti-ESCC agent. Romidepsin treatment decreased cell viability, induced apoptosis and cell cycle arrest in ESCC cell lines, and these findings were confirmed in ESCC cell line-derived xenografted (CDX) mouse models. Mechanically, romidepsin induced transcriptional upregulation of DNA damage-inducible transcript 4 (DDIT4) gene by histone hyperacetylation at its promoter region, leading to the inhibition of mammalian target of rapamycin complex 1 (mTORC1) pathway. Furthermore, romidepsin exhibited better efficacy and safety compared to the conventional therapeutic drugs in ESCC patient-derived xenografted (PDX) mouse models. These data indicate that romidepsin may be a novel option for anti-ESCC therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Correspondence and requests for materials should be addressed to LX-W (lxwu@cqmu.edu.cn).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res. 2019;144:95–135. https://doi.org/10.1016/bs.acr.2019.05.004.
Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88:413–26. https://doi.org/10.1016/j.gie.2018.04.2352.
Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 2019;49:412–20. https://doi.org/10.1093/jjco/hyz034.
Kondo J, Inoue M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells. 2019;8:470. https://doi.org/10.3390/cells8050470.
Lee DW, Lee SY, Doh I, Ryu GH, Nam DH. High-Dose Compound Heat Map for 3D-Cultured Glioblastoma Multiforme Cells in a Micropillar and Microwell Chip Platform. Biomed Res Int. 2017;2017:7218707. https://doi.org/10.1155/2017/7218707.
Yan X, Zhou L, Wu Z, Wang X, Chen X, Yang F, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing. Biomaterials. 2019;198:167–79. https://doi.org/10.1016/j.biomaterials.2018.05.020.
Huang Y, Dai Y, Wen C, He S, Shi J, Zhao D, et al. circSETD3 Contributes to Acquired Resistance to Gefitinib in Non-Small-Cell Lung Cancer by Targeting the miR-520h/ABCG2 Pathway. Mol Ther Nucleic Acids. 2020;21:885–99. https://doi.org/10.1016/j.omtn.2020.07.027.
Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91. https://doi.org/10.1200/JCO.2010.28.9066.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. https://doi.org/10.1177/108705719900400206.
Thuss-Patience P, Stein A. Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr Oncol. 2022;29:2461–71. https://doi.org/10.3390/curroncol29040200.
Thuru X, Magnez R, El-Bouazzati H, Vergoten G, Quesnel B, Bailly C. Drug Repurposing to Enhance Antitumor Response to PD-1/PD-L1 Immune Checkpoint Inhibitors. Cancers. 2022;14:3368. https://doi.org/10.3390/cancers14143368.
Foltyn M, Luger AL, Lorenz NI, Sauer B, Mittelbronn M, Harter PN, et al. The physiological mTOR complex 1 inhibitor DDIT4 mediates therapy resistance in glioblastoma. Br J Cancer. 2019;120:481–7. https://doi.org/10.1038/s41416-018-0368-3.
El Omari N, Lee LH, Bakrim S, Makeen HA, Alhazmi HA, Mohan S, et al. Molecular mechanistic pathways underlying the anticancer therapeutic efficiency of romidepsin. Biomed Pharmacother. 2023;164:114774. https://doi.org/10.1016/j.biopha.2023.114774.
Wang Y, Han E, Xing Q, Yan J, Arrington A, Wang C, et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 2015;358:170–9. https://doi.org/10.1016/j.canlet.2014.12.033.
Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191–201. https://doi.org/10.1038/onc.2016.363.
Zeng M, Ruan Z, Tang J, Liu M, Hu C, Fan P, et al. Generation, evolution, interfering factors, applications, and challenges of patient-derived xenograft models in immunodeficient mice. Cancer Cell Int. 2023;23:120. https://doi.org/10.1186/s12935-023-02953-3.
Rivers ZT, Oostra DR, Westholder JS, Vercellotti GM. Romidepsin-associated cardiac toxicity and ECG changes: A case report and review of the literature. J Oncol Pharm Pr. 2018;24:56–62. https://doi.org/10.1177/1078155216673229.
Shi Y, Fu Y, Zhang X, Zhao G, Yao Y, Guo Y, et al. Romidepsin (FK228) regulates the expression of the immune checkpoint ligand PD-L1 and suppresses cellular immune functions in colon cancer. Cancer Immunol Immunother. 2021;70:61–73. https://doi.org/10.1007/s00262-020-02653-1.
Jiang YY, Jiang Y, Li CQ, Zhang Y, Dakle P, Kaur H, et al. TP63, SOX2, and KLF5 Establish a Core Regulatory Circuitry That Controls Epigenetic and Transcription Patterns in Esophageal Squamous Cell Carcinoma Cell Lines. Gastroenterology. 2020;159:1311–e19.
Hoshino I, Matsubara H, Akutsu Y, Nishimori T, Yoneyama Y, Murakami K, et al. Gene expression profiling induced by histone deacetylase inhibitor, FK228, in human esophageal squamous cancer cells. Oncol Rep. 2007;18:585–92.
Hoshino I, Matsubara H, Hanari N, Mori M, Nishimori T, Yoneyama Y, et al. Histone deacetylase inhibitor FK228 activates tumor suppressor Prdx1 with apoptosis induction in esophageal cancer cells. Clin Cancer Res. 2005;11:7945–52. https://doi.org/10.1158/1078-0432.CCR-05-0840.
Ahrens TD, Timme S, Hoeppner J, Ostendorp J, Hembach S, Follo M, et al. Selective inhibition of esophageal cancer cells by combination of HDAC inhibitors and Azacytidine. Epigenetics. 2015;10:431–45. https://doi.org/10.1080/15592294.2015.1039216.
Shah RR. Safety and Tolerability of Histone Deacetylase (HDAC) Inhibitors in Oncology. Drug Saf. 2019;42:235–45. https://doi.org/10.1007/s40264-018-0773-9.
Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol. 2002;22:2283–93. https://doi.org/10.1128/MCB.22.7.2283-2293.2002.
Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell. 2002;10:995–1005. https://doi.org/10.1016/s1097-2765(02)00706-2.
Zhidkova EM, Lylova ES, Grigoreva DD, Kirsanov KI, Osipova AV, Kulikov EP, et al. Nutritional Sensor REDD1 in Cancer and Inflammation: Friend or Foe? Int J Mol Sci. 2022;23:9686. https://doi.org/10.3390/ijms23179686.
Ding F, Gao F, Zhang S, Lv X, Chen Y, Liu Q. A review of the mechanism of DDIT4 serve as a mitochondrial related protein in tumor regulation. Sci Prog. 2021;104:36850421997273. https://doi.org/10.1177/0036850421997273.
Tirado-Hurtado I, Fajardo W, Pinto JA. DNA Damage Inducible Transcript 4 Gene: The Switch of the Metabolism as Potential Target in Cancer. Front Oncol. 2018;8:106. https://doi.org/10.3389/fonc.2018.00106.
Song L, Chen Z, Zhang M, Zhang M, Lu X, Li C, et al. DDIT4 overexpression associates with poor prognosis in lung adenocarcinoma. J Cancer. 2021;12:6422–8. https://doi.org/10.7150/jca.60118.
Zhang Z, Zhu H, Zhao C, Liu D, Luo J, Ying Y, et al. DDIT4 promotes malignancy of head and neck squamous cell carcinoma. Mol Carcinog. 2023;62:332–47. https://doi.org/10.1002/mc.23489.
Koo JS, Jung W. Alteration of REDD1-mediated mammalian target of rapamycin pathway and hypoxia-inducible factor-1alpha regulation in human breast cancer. Pathobiology. 2010;77:289–300. https://doi.org/10.1159/000320936.
Peng X, Yang R, Peng W, Zhao Z, Tu G, He B, et al. Overexpression of LINC00551 promotes autophagy-dependent ferroptosis of lung adenocarcinoma via upregulating DDIT4 by sponging miR-4328. PeerJ. 2022;10:e14180. https://doi.org/10.7717/peerj.14180.
Hu T, Wang F, Han G. LncRNA PSMB8-AS1 acts as ceRNA of miR-22-3p to regulate DDIT4 expression in glioblastoma. Neurosci Lett. 2020;728:134896. https://doi.org/10.1016/j.neulet.2020.134896.
Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, et al. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–74. https://doi.org/10.1038/onc.2010.618.
Chen W, Chen Y, Wu R, Guo G, Liu Y, Zeng B, et al. DHA alleviates diet-induced skeletal muscle fiber remodeling via FTO/m(6)A/DDIT4/PGC1alpha signaling. BMC Biol. 2022;20:39. https://doi.org/10.1186/s12915-022-01239-w.
Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71. https://doi.org/10.1186/s13045-019-0754-1.
Zhang S, Lin X, Hou Q, Hu Z, Wang Y, Wang Z. Regulation of mTORC1 by amino acids in mammalian cells: A general picture of recent advances. Anim Nutr. 2021;7:1009–23. https://doi.org/10.1016/j.aninu.2021.05.003.
Li Y, Tao L, Zuo Z, Zhou Y, Qian X, Lin Y, et al. ZY0511, a novel, potent and selective LSD1 inhibitor, exhibits anticancer activity against solid tumors via the DDIT4/mTOR pathway. Cancer Lett. 2019;454:179–90. https://doi.org/10.1016/j.canlet.2019.03.052.
Liao KF, Chiu TL, Huang SY, Hsieh TF, Chang SF, Ruan JW, et al. Anti-Cancer Effects of Radix Angelica Sinensis (Danggui) and N-Butylidenephthalide on Gastric Cancer: Implications for REDD1 Activation and mTOR Inhibition. Cell Physiol Biochem. 2018;48:2231–46. https://doi.org/10.1159/000492641.
Kim TS, Lee M, Park M, Kim SY, Shim MS, Lee CY, et al. Metformin and Dichloroacetate Suppress Proliferation of Liver Cancer Cells by Inhibiting mTOR Complex 1. Int J Mol Sci. 2021;22:10027. https://doi.org/10.3390/ijms221810027.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20:206. https://doi.org/10.1186/s12967-022-03405-8.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 82073938, 82274023, 82373135), Youth Innovation in Future Medicine, Chongqing Medical University (No. W0093), and the Scientific and Technological Research Program of Chongqing Municipal Education Commission (No. KJQN202200432).
Author information
Authors and Affiliations
Contributions
WFX: Methodology, Formal analysis, Investigation, Writing – original draft. XLZ: Methodology, Formal analysis, Investigation. WYL: Methodology, Resources. YTH: Formal analysis, Investigation. CJW: Investigation. HHZ: Data interpretation. QCW: Project administration, Supervision. LXW: Writing-review & editing, Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, WF., Zheng, XL., Liu, WY. et al. Romidepsin exhibits anti-esophageal squamous cell carcinoma activity through the DDIT4-mTORC1 pathway. Cancer Gene Ther (2024). https://doi.org/10.1038/s41417-024-00760-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41417-024-00760-0