Abstract
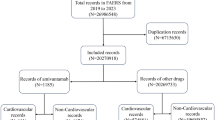
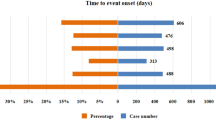
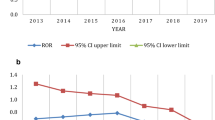
Chimeric antigen receptor T-cell (CAR-T) therapy has achieved durable response in patients with hematological malignancies, however, therapy-associated multisystem toxicities are commonly observed. Here, we systematically analyzed CAR-T-related gastrointestinal adverse events (GAEs) using the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) between January 2017 and December 2021. Disproportionality analyses were performed using reporting odds ratios (ROR) and information component (IC). Among 105,087,611 reports in FAERS, 1518 CAR-T-related GAEs reports were identified. 23 GAEs (n = 281, 18.51%) were significantly overreported following CAR-T therapy compared with the full database, of which 11 GAEs (n = 156, 10.28%) were associated with gastrointestinal infections (GI), such as clostridium difficile colitis (n = 44 [2.90%], ROR = 5.55), enterovirus infection (n = 23 [1.52%], ROR = 20.02), and mucormycosis (n = 15 [0.99%], ROR = 3.09). Overall, the fatality rate of 11 GI-related AEs was 29.49%, especially mucormycosis causing substantial mortality with 60%. In addition, 4 of 23 overreported GAEs were related to haemorrhage and the mortality of gastrointestinal haemorrhage was 73.17%. Lastly, 29 death-related GAEs were identified. These findings could help clinicians early alert those rarely reported but lethal GAEs, thus reducing the risk of severe toxicities.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
This study was performed based on the data from Food and Drug Administration adverse event reporting system (FAERS), a publicly available and anonymized database (https://www.fda.gov/regulatory-information/freedom-information).
References
Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 2021;39:1650–9.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene Ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119–28.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell Lymphoma. N. Engl J Med. 2019;380:45–56.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell Lymphoblastic Leukemia. N. Engl J Med. 2018;378:439–48.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA−1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502.
Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018;132:777–81.
Sadeqi Nezhad M, Yazdanifar M, Abdollahpour-Alitappeh M, Sattari A, Seifalian A, Bagheri N. Strengthening the CAR-T cell therapeutic application using CRISPR/Cas9 technology. Biotechnol Bioeng. 2021;118:3691–705.
Tbakhi B, Reagan PM. Chimeric antigen receptor (CAR) T-cell treatment for mantle cell lymphoma (MCL). Ther Adv Hematol. 2022;13:20406207221080738.
Frey NV. Approval of brexucabtagene autoleucel for adults with relapsed and refractory acute lymphocytic leukemia. Blood. 2022;140:11−5.
Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28:713–23.
Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Hematol. 2019;94:S42–S49.
Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–71.
Uribe-Herranz M, Beghi S, Ruella M, Parvathaneni K, Salaris S, Kostopoulos N, et al. Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy. Mol Ther. 2023;31:686–700.
Bureš J, Kohoutová D, Zavoral M. Gastrointestinal toxicity of systemic oncology immunotherapy. Klin Onkol. 2022;35:346–57.
Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403−15.
Mahmood SS, Riedell PA, Feldman S, George G, Sansoterra SA, Althaus T, et al. Biomarkers and cardiovascular outcomes in chimeric antigen receptor T-cell therapy recipients. Eur Heart J. 2023;44:2029–42.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109−17.
Ang PS, Chen Z, Chan CL, Tai BC. Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin Drug Saf. 2016;15:583–90.
Hou Y, Ye X, Wu G, Cheng G, Du X, He J. A comparison of disproportionality analysis methods in national adverse drug reaction databases of China. Expert Opin Drug Saf. 2014;13:853–7.
Song Z, Tu D, Tang G, Liu N, Tai Z, Yang J, et al. Hemophagocytic lymphohistiocytosis and disseminated intravascular coagulation are underestimated, but fatal adverse events in chimeric antigen receptor T-cell therapy. Haematologica. 2023;108:2067–79.
Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22:57–69.
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Wang Y, Qi K, Cheng H, Cao J, Shi M, Qiao J, et al. Coagulation disorders after chimeric antigen receptor T cell therapy: analysis of 100 patients with relapsed and refractory hematologic malignancies. Biol Blood Marrow Transpl. 2020;26:865–75.
Saez-Ibañez AR, Upadhaya S, Partridge T, Shah M, Correa D, Campbell J. Landscape of cancer cell therapies: trends and real-world data. Nat Rev Drug Discov. 2022;21:631–2.
Cai C, Tang D, Han Y, Shen E, Ahmed OA, Guo C, et al. A comprehensive analysis of the fatal toxic effects associated with CD19 CAR-T cell therapy. Aging (Albany NY). 2020;12:18741–53.
Abu-Sbeih H, Tang T, Ali FS, Luo W, Neelapu SS, Westin JR, et al. Gastrointestinal adverse events observed after chimeric antigen receptor T-cell therapy. Am J Clin Oncol. 2019;42:789–96.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl J Med. 2017;377:2531–44.
Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA−1, and TRANSCEND trials. Am J Hematol. 2021;96:1295–312.
Brudno JN, Natrakul D, Lam N, Dulau-Florea A, Yuan CM, Kochenderfer JN. Acute and delayed cytopenias following CAR T-cell therapy: an investigation of risk factors and mechanisms. Leuk Lymphoma. 2022;63:1849–60.
Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 2019;54:1643–50.
Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–35.
Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13:5313.
Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39:3978–92.
Ali S, Kjeken R, Niederlaender C, Markey G, Saunders TS, Opsata M, et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25:e321–e327.
Philipson BI, O’Connor RS, May MJ, June CH, Albelda SM, Milone MC. 4−1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci Signal. 2020;13:eaay8248.
Kyriakidis I, Mantadakis E, Stiakaki E, Groll AH, Tragiannidis A. Infectious complications of targeted therapies in children with leukemias and lymphomas. Cancers. 2022;14:5022.
Fusaroli M, Isgrò V, Cutroneo PM, Ferrajolo C, Cirillo V, Del Bufalo F, et al. Post-marketing surveillance of CAR-T-cell therapies: analysis of the FDA Adverse Event Reporting System (FAERS) Database. Drug Saf. 2022;45:891–908.
Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37:48–52.
Li Y, Ming Y, Fu R, Li C, Wu Y, Jiang T, et al. The pathogenesis, diagnosis, prevention, and treatment of CAR-T cell therapy-related adverse reactions. Front Pharm. 2022;13:950923.
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Acknowledgements
The authors thank all the participants and information uploaders in the FAERS.
Funding
J Yang received funding from the National Natural Science Foundation of China (81770209, 82270202) and Shanghai 2021 “Action Plan of Technological Innovation” Biomedical Science and Technology Support Special Project (21S11906100). J Chen received funding from the National Natural Science Foundation of China (81970178). N Liu received funding from the National Natural Science Foundation of China (82100162). Y Wang received funding from the National Natural Science Foundation of China (82300257). Y Wang received funding from the Youth Start-up Foundation of the First Affiliated Hospital of Second Military Medical University (2020QNB03, 2022QN067).
Author information
Authors and Affiliations
Contributions
Na Liu, Jie Chen, Jianmin Yang: Conceptualization, Methodology, Software; Zhiqiang Song, Yang Wang, Ping Liu: Data curation, Visualization, Investigation, Writing-Original draft preparation; Jianmin Yang: Supervision; Zhiqiang Song, Yang Wang, Yuke Geng: Software, Validation; Na Liu, Jie Chen, Jianmin Yang: Writing-Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Because the data in FAERS is anonymous and publicly available, the requirement to obtain informed consent and Institutional Review Board approval were waived.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Z., Wang, Y., Liu, P. et al. Gastrointestinal infections and gastrointestinal haemorrhage are underestimated but serious adverse events in chimeric antigen receptor T-cell recipients: A real-world study. Cancer Gene Ther (2024). https://doi.org/10.1038/s41417-024-00752-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41417-024-00752-0