Abstract
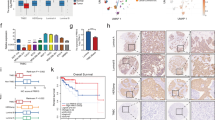
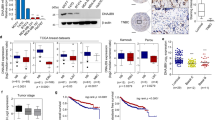
TNFRSF19 is a member of the tumor necrosis factor receptor superfamily, and its function exhibits variability among different types of cancers. The influence of TNFRSF19 on triple-negative breast cancer (TNBC) has yet to be definitively established. In this study, bioinformatics analyses revealed that lower TNFRSF19 was associated with the poorer prognosis, higher lymph node metastasis and lower immune infiltration. Subsequently, data obtained from the TCGA database and collection of tissue samples revealed that the mRNA and protein expression levels of TNFRSF19 were observed to be significantly reduced in TNBC tissue compared to normal tissue. Additionally, the results of in vitro experiments have demonstrated that TNFRSF19 possessed the ability to inhibit the proliferation, migration and invasive capabilities of TNBC cells. In vivo trials elucidated that TNFRSF19 could suppress tumor xenografts growth. Mechanistically, TNFRSF19 initiated caspase-independent cell death and induced paraptosis. Moreover, rescue assays demonstrated that TNFRSF19 induced-paraptosis was facilitated by MAPK pathway-mediated endoplasmic reticulum (ER) stress. In conclusion, our findings demonstrated that the upregulation of TNFRSF19 functioned as a tumor suppressor in TNBC by stimulating paraptosis through the activation of the MAPK pathway-mediated ER stress, highlighting its potential to be a new therapeutic target for TNBC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the data generated or analyzed during the study are included in this article or are available upon request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Won KA, Spruck C. Triple‑negative breast cancer therapy: current and future perspectives (Review). Int J Oncol. 2020;57:1245–61.
Kojima T, Morikawa Y, Copeland NG, Gilbert DJ, Jenkins NA, Senba E, et al. TROY, a newly identified member of the tumor necrosis factor receptor superfamily, exhibits a homology with Edar and is expressed in embryonic skin and hair follicles. J Biol Chem. 2000;275:20742–7.
Eby MT, Jasmin A, Kumar A, Sharma K, Chaudhary PM. TAJ, a novel member of the tumor necrosis factor receptor family, activates the c-Jun N-terminal kinase pathway and mediates caspase-independent cell death. J Biol Chem. 2000;275:15336–42.
Dostert C, Grusdat M, Letellier E, Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev. 2019;99:115–60.
Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, et al. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11:1066–75.
Wang Y, Wen X, Zhang N, Wang L, Hao D, Jiang X, et al. Small-molecule compounds target paraptosis to improve cancer therapy. Biomed Pharmacother. 2019;118:109203.
Wang L, Gundelach JH, Bram RJ. Cycloheximide promotes paraptosis induced by inhibition of cyclophilins in glioblastoma multiforme. Cell Death Dis. 2017;8:e2807.
Lee D, Kim IY, Saha S, Choi KS. Paraptosis in the anti-cancer arsenal of natural products. Pharm Ther. 2016;162:120–33.
Ram BM, Ramakrishna G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine A treated cancer cervix cells is mediated by cyclophilin B inhibition. Biochim Biophys Acta. 2014;1843:2497–512.
Hoa N, Myers MP, Douglass TG, Zhang JG, Delgado C, Driggers L, et al. Molecular mechanisms of paraptosis induction: implications for a non-genetically modified tumor vaccine. PLoS One. 2009;4:e4631.
Chung AH, Leisner TM, Dardis GJ, Bivins MM, Keller AL, Parise LV. CIB1 depletion with docetaxel or TRAIL enhances triple-negative breast cancer cell death. Cancer Cell Int. 2019;19:26.
Wang Y, Li X, Wang L, Ding P, Zhang Y, Han W, et al. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci. 2004;117:1525–32.
Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–16.e11.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34.
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–8.
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173:338–54.e15.
Pizato N, Luzete BC, Kiffer L, Correa LH, de Oliveira Santos I, Assumpcao JAF, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952.
Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25:8159–68.
Ekstrand J, Zemmler M, Abrahamsson A, Lundberg P, Forsgren M, Dabrosin C. Breast density and estradiol are major determinants for soluble TNF-TNF-R proteins in vivo in human breast tissue. Front Immunol. 2022;13:850240.
Guo L, Gao R, Gan J, Zhu Y, Ma J, Lv P, et al. Downregulation of TNFRSF19 and RAB43 by a novel miRNA, miR-HCC3, promotes proliferation and epithelial-mesenchymal transition in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2020;525:425–32.
Wilhelm F, Boger C, Kruger S, Behrens HM, Rocken C. Troy is expressed in human stomach mucosa and a novel putative prognostic marker of intestinal type gastric cancer. Oncotarget. 2017;8:50557–69.
Shao L, Zuo X, Yang Y, Zhang Y, Yang N, Shen B, et al. The inherited variations of a p53-responsive enhancer in 13q12.12 confer lung cancer risk by attenuating TNFRSF19 expression. Genome Biol. 2019;20:103.
Spanjaard RA, Whren KM, Graves C, Bhawan J. Tumor necrosis factor receptor superfamily member TROY is a novel melanoma biomarker and potential therapeutic target. Int J Cancer. 2007;120:1304–10.
Ding Z, Dhruv H, Kwiatkowska-Piwowarczyk A, Ruggieri R, Kloss J, Symons M, et al. PDZ-RhoGEF is a signaling effector for TROY-induced glioblastoma cell invasion and survival. Neoplasia. 2018;20:1045–58.
Paulino VM, Yang Z, Kloss J, Ennis MJ, Armstrong BA, Loftus JC, et al. TROY (TNFRSF19) is overexpressed in advanced glial tumors and promotes glioblastoma cell invasion via Pyk2-Rac1 signaling. Mol Cancer Res. 2010;8:1558–67.
Ding Z, Roos A, Kloss J, Dhruv H, Peng S, Pirrotte P, et al. A novel signaling complex between TROY and EGFR mediates glioblastoma cell invasion. Mol Cancer Res. 2018;16:322–32.
Deng C, Lin YX, Qi XK, He GP, Zhang Y, Zhang HJ, et al. TNFRSF19 inhibits TGFbeta signaling through interaction with TGFbeta receptor type I to promote tumorigenesis. Cancer Res. 2018;78:3469–83.
Saberi S, Piryaei A, Mirabzadeh E, Esmaeili M, Karimi T, Momtaz S, et al. Immunohistochemical analysis of LGR5 and TROY expression in gastric carcinogenesis demonstrates an inverse trend. Iran Biomed J. 2019;23:107–20.
Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res. 2006;4:667–81.
Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52.
Li GN, Zhao XJ, Wang Z, Luo MS, Shi SN, Yan DM, et al. Elaiophylin triggers paraptosis and preferentially kills ovarian cancer drug-resistant cells by inducing MAPK hyperactivation. Signal Transduct Target Ther. 2022;7:317.
Hong A, Moriceau G, Sun L, Lomeli S, Piva M, Damoiseaux R, et al. Exploiting drug addiction mechanisms to select against MAPKi-resistant melanoma. Cancer Discov. 2018;8:74–93.
Yoon MJ, Kim EH, Kwon TK, Park SA, Choi KS. Simultaneous mitochondrial Ca(2+) overload and proteasomal inhibition are responsible for the induction of paraptosis in malignant breast cancer cells. Cancer Lett. 2012;324:197–209.
Acknowledgements
All authors are grateful to each participant involved in this study. We appreciate the support from two pathologists (Dr. Ge Wang and Dr. Shengwei Mo).
Funding
This research was funded by National Natural Science Foundation of China (Grant No 21834002).
Author information
Authors and Affiliations
Contributions
SL: analyzed data, performed software, in vitro/in vivo trials and draft the manuscript. YT: reviewing and in vivo trials. CL: visualization. TY and ZG: data collection and figure preparation. LZ: conceptualization, discussed analysis and resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology granted approval for all animal experiments. The Ethics Committee of Wuhan Tongji Hospital granted approval for the collection of patients’ samples, and informed consent forms were obtained from the patients prior to sample collection.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Tian, Y., Liu, C. et al. TNFRSF19 promotes endoplasmic reticulum stress-induced paraptosis via the activation of the MAPK pathway in triple-negative breast cancer cells. Cancer Gene Ther 31, 217–227 (2024). https://doi.org/10.1038/s41417-023-00696-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00696-x