Abstract
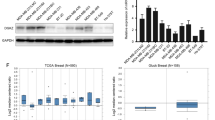
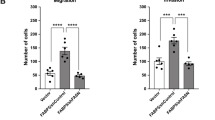
Tamoxifen is the frontline therapeutic agent for the estrogen receptor-positive (ER + ) subtype of breast cancer patients, which accounts for 70–80% of total breast cancer incidents. However, clinical resistance to tamoxifen has become increasingly common, highlighting the need to identify the underlying cellular mechanisms. In our study, we employed a genome-scale CRISPR-Cas9 loss-of-function screen and validation experiments to discover that Tafazzin (TAZ), a mitochondrial transacylase, is crucial for maintaining the cellular sensitivity of ER+ breast cancer cells to tamoxifen and other chemotherapies. Mechanistically, we found that cardiolipin, whose synthesis and maturation rely on TAZ, is required to maintain cellular sensitivity to tamoxifen. Loss of metabolic enzymatic activity of TAZ causes ERα downregulation and therapy resistance. Interestingly, we observed that TAZ deficiency also led to the upregulation of lysophosphatidylcholine (LPC), which in turn suppressed ERα expression and nuclear localization, thereby contributing to tamoxifen resistance. LPC is further metabolized to lysophosphatidic acid (LPA), a bioactive molecule that supports cell survival. Thus, our findings suggest that the depletion of TAZ promotes tamoxifen resistance through an LPC-LPA phospholipid synthesis axis, and targeting this lipid metabolic pathway could restore cell susceptibility to tamoxifen treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Additional data could be reasonably requested from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584–90.
Kittaneh M, Montero AJ, Gluck S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013;5:61–70.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66.
Giuliano M, Schiff R, Osborne CK, Trivedi MV. Biological mechanisms and clinical implications of endocrine resistance in breast cancer. Breast. 2011;20:S42–S49.
Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharm Ther. 2018;186:1–24.
Droog M, Beelen K, Linn S, Zwart W. Tamoxifen resistance: from bench to bedside. Eur J Pharm. 2013;717:47–57.
Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pr Res Clin Endocrinol Metab. 2015;29:557–68.
Yao J, Deng K, Huang J, Zeng R, Zuo J. Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer. Front Pharm. 2020;11:592912.
Ranganathan P, Nadig N, Nambiar S. Non-canonical Estrogen Signaling in Endocrine Resistance. Front Endocrinol (Lausanne). 2019;10:708.
Mendes-Pereira AM, Sims D, Dexter T, Fenwick K, Assiotis I, Kozarewa I, et al. Genome-wide functional screen identifies a compendium of genes affecting sensitivity to tamoxifen. Proc Natl Acad Sci USA. 2012;109:2730–5.
Ahn BY, Elwi AN, Lee B, Trinh DL, Klimowicz AC, Yau A, et al. Genetic screen identifies insulin-like growth factor binding protein 5 as a modulator of tamoxifen resistance in breast cancer. Cancer Res. 2010;70:3013–9.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Katti A, Diaz BJ, Caragine CM, Sanjana NE, Dow LE. CRISPR in cancer biology and therapy. Nat Rev Cancer. 2022;22:259–79.
Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE, et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat Genet. 2020;52:198–207.
Schlame M, Xu Y. The Function of Tafazzin, a Mitochondrial Phospholipid-Lysophospholipid Acyltransferase. J Mol Biol. 2020;432:5043–51.
Seneviratne AK, Xu M, Henao JJA, Fajardo VA, Hao Z, Voisin V, et al. The Mitochondrial Transacylase, Tafazzin, Regulates for AML Stemness by Modulating Intracellular Levels of Phospholipids. Cell Stem Cell. 2019;24:621–36.e616.
Lu YW, Galbraith L, Herndon JD, Lu YL, Pras-Raves M, Vervaart M, et al. Defining functional classes of Barth syndrome mutation in humans. Hum Mol Genet. 2016;25:1754–70.
Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells. 2019;8:728.
Claypool SM, McCaffery JM, Koehler CM. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J Cell Biol. 2006;174:379–90.
Samanta K, Chakravarti B, Mishra JK, Dwivedi SK, Nayak LV, Choudhry P, et al. Anti-tumor activity of a new series of benzoxazepine derivatives in breast cancer. Bioorg Med Chem Lett. 2010;20:283–7.
Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34:184–91.
Joung J, Konermann S, Gootenberg JS, Abudayyeh OO, Platt RJ, Brigham MD, et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12:828–63.
Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15:554.
Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168.
Garcia-Martinez L, Zhang Y, Nakata Y, Chan HL, Morey L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat Commun. 2021;12:1786.
Diaz Bessone MI, Gattas MJ, Laporte T, Tanaka M, Simian M. The Tumor Microenvironment as a Regulator of Endocrine Resistance in Breast Cancer. Front Endocrinol (Lausanne). 2019;10:547.
Hanker AB, Sudhan DR, Arteaga CL. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37:496–513.
Ahmadpour ST, Maheo K, Servais S, Brisson L, Dumas JF. Cardiolipin, the Mitochondrial Signature Lipid: Implication in Cancer. Int J Mol Sci. 2020;21:8031.
Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20:398–411.
Guler GD, Tindell CA, Pitti R, Wilson C, Nichols K, KaiWai Cheung T, et al. Repression of Stress-Induced LINE-1 Expression Protects Cancer Cell Subpopulations from Lethal Drug Exposure. Cancer Cell. 2017;32:221–37.e213.
Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell. 2017;20:233–46.e237.
Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–96.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87.
Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, et al. A Conserved Circular Network of Coregulated Lipids Modulates Innate Immune Responses. Cell. 2015;162:170–83.
Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int J Mol Sci. 2019;20:1149.
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–33.
Brindley DN, Lin FT, Tigyi GJ. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim Biophys Acta. 2013;1831:74–85.
Rani A, Stebbing J, Giamas G, Murphy J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front Endocrinol (Lausanne). 2019;10:245.
Ingle JN, Mailliard JA, Schaid DJ, Krook JE, Gesme DH Jr, Windschitl HE, et al. A double-blind trial of tamoxifen plus prednisolone versus tamoxifen plus placebo in postmenopausal women with metastatic breast cancer. A collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic. Cancer. 1991;68:34–9.
Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995;13:513–29.
Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39:759–78.
Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;19:6389–97.
Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–22.
Massague J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021;11:971–94.
Rehman SK, Haynes J, Collignon E, Brown KR, Wang Y, Nixon AML, et al. Colorectal Cancer Cells Enter a Diapause-like DTP State to Survive Chemotherapy. Cell. 2021;184:226–42.e221.
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80.
Ahn E-Y, Pan G, Oh JH, Tytler EM, McDonald JM. The Combination of Calmodulin Antagonists and Interferonγ Induces Apoptosis through CaspaseDependent and -Independent Pathways in Cholangiocarcinoma Cells. Am J Pathol. 2003;163:2053–63.
Pawar P, Ma L, Byon CH, Liu H, Ahn EY, Jhala N, et al. Molecular mechanisms of tamoxifen therapy for cholangiocarcinoma: role of calmodulin. Clin Cancer Res. 2009;15:1288–96.
Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61:6938–44.
Yang SY, Lee J, Park CG, Kim S, Hong S, Chung HC, et al. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis. 2002;19:603–8.
Brindley DN, Tang X, Meng G, Benesch MGK. Role of Adipose Tissue-Derived Autotaxin, Lysophosphatidate Signaling, and Inflammation in the Progression and Treatment of Breast Cancer. Int J Mol Sci. 2020;21:5938.
Chae CS, Sandoval TA, Hwang SM, Park ES, Giovanelli P, Awasthi D, et al. Tumor-Derived Lysophosphatidic Acid Blunts Protective Type I Interferon Responses in Ovarian Cancer. Cancer Discov. 2022;12:1904–21.
Tan Z, Lei H, Guo M, Chen Y, Zhai X. An updated patent review of autotaxin inhibitors (2017-present). Expert Opin Ther Pat. 2021;31:421–34.
Zulfikar S, Mulholland S, Adamali H, Barratt SL. Inhibitors of the Autotaxin-Lysophosphatidic Acid Axis and Their Potential in the Treatment of Interstitial Lung Disease: Current Perspectives. Clin Pharm. 2020;12:97–108.
Acknowledgements
We thank D. Pan for helping with MAGeCK analysis; Z. Chang and Q. Ye for generously sharing the tamoxifen resistance cell; We thank all members of the Zheng laboratory for helpful discussions and technical assistance. We thank the Technology Center for Protein Sciences at Tsinghua University for mass-spectrometry support, the Laboratory Animal Research Center for animal research support, and the Center of Biomedical Analysis at Tsinghua University for FACS and optical imaging support.
Funding
The study was partially supported by the National Key Research and Development Program of China (2020YFA0509400 to HZ), the National Science Foundation of China (81772981 and 81972462 to HZ), the Tsinghua University Initiative Scientific Research Program, and the Tsinghua-Peking Center for Life Sciences.
Author information
Authors and Affiliations
Contributions
XL and HZ designed the overall study. XL performed the experiments, analyzed data, and wrote the paper. TZ provided technical help for next-generation data analysis. LZ provided help with the CRISPR/Cas9 screening. YZ provided technical help for animal work. HH provided technical help for cell works. HZ supervised the overall study. HZ, CL, and XL revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Tsinghua University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhang, Y., Zhang, T. et al. Tafazzin mediates tamoxifen resistance by regulating cellular phospholipid composition in ER-positive breast cancer. Cancer Gene Ther 31, 69–81 (2024). https://doi.org/10.1038/s41417-023-00683-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00683-2