Abstract
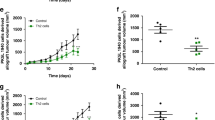
Although it can promote effector T-cell function, the summative effect of interleukin-10 (IL-10) in the tumor microenvironment (TME) appears to be suppressive; therefore, blocking this critical regulatory cytokine has therapeutic potential to enhance antitumor immune function. As macrophages efficiently localize to the TME, we hypothesized that they could be used as a delivery vehicle for drugs designed to block this pathway. To test our hypothesis, we created and evaluated genetically engineered macrophages (GEMs) that produce an IL-10-blocking antibody (αIL-10). Healthy donor human peripheral blood mononuclear cells were differentiated and transduced with a novel lentivirus (LV) encoding BT-063, a humanized αIL-10 antibody. The efficacy of αIL-10 GEMs was assessed in human gastrointestinal tumor slice culture models developed from resected specimens of pancreatic ductal adenocarcinoma primary tumors and colorectal cancer liver metastases. LV transduction led to sustained production of BT-063 by αIL-10 GEMs for at least 21 days. Transduction did not alter GEM phenotype as evaluated by flow cytometry, but αIL-10 GEMs produced measurable quantities of BT-063 in the TME that was associated with an ~5-fold higher rate of tumor cell apoptosis than control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as online Supplementary Information. Source data will be made available upon direct request to the corresponding author.
References
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Wu AA, Jaffee E, Lee V. Current status of immunotherapies for treating pancreatic cancer. Curr Oncol Rep. 2019;21:60.
Spranger S, Gajewski TF. Mechanisms of tumor cell–intrinsic immune evasion. Annu Rev Cancer Biol. 2018;2:213–28.
Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51:170–82.
Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367:103–7.
Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, et al. Interleukin-10 directly inhibits CD8+T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity. 2018;48:299–312.e5.
Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, et al. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34:775–91.e3.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho Christine MT, Pryer N, et al. Macrophage IL-10 blocks CD8+T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37.
Shen L, Li J, Liu Q, Song W, Zhang X, Tiruthani K, et al. Local blockade of interleukin 10 and C-X-C motif chemokine ligand 12 with nano-delivery promotes antitumor response in murine cancers. ACS Nano. 2018;12:9830–41.
Chen S, Ni G, Wu X, Zhu B, Liao Z, Wang Y, et al. Blocking IL-10 signalling at the time of immunization renders the tumour more accessible to T cell infiltration in mice. Cell Immunol. 2016;300:9–17.
Ni G, Zhang L, Yang X, Li H, Ma B, Walton S, et al. Targeting interleukin-10 signalling for cancer immunotherapy, a promising and complicated task. Hum Vaccin Immunother. 2020;16:2328–32.
Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–51.
Sullivan KM, Jiang X, Guha P, Lausted C, Carter JA, Hsu C, et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2022;72:325–37.
Lee S, Kivimäe S, Dolor A, Szoka FC. Macrophage-based cell therapies: the long and winding road. J Control Release. 2016;240:527–40.
Burke B, Sumner S, Maitland N, Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J Leukoc Biol. 2002;72:417–28.
Stevenson HC, Keenan AM, Woodhouse C, Ottow RT, Miller P, Steller EP, et al. Fate of gamma-interferon-activated killer blood monocytes adoptively transferred into the abdominal cavity of patients with peritoneal carcinomatosis. Cancer Res. 1987;47:6100–3.
Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol. 1998;64:419–26.
Brempelis KJ, Cowan CM, Kreuser SA, Labadie KP, Prieskorn BM, Lieberman NAP, et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J Immunother Cancer. 2020;8:e001356.
Moyes KW, Lieberman NAP, Kreuser SA, Chinn H, Winter C, Deutsch G, et al. Genetically engineered macrophages: a potential platform for cancer immunotherapy. Hum Gene Ther. 2017;28:200–15.
Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–8.
Bobadilla S, Sunseri N, Landau NR. Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene Ther. 2013;20:514–20.
Osterroth F, Uherek C, Bruecher C, Röttgen P, Daelken B, Engling A, et al., inventors; Biotest AG, assignee. Humanized anti-IL 10 antibodies for the treatment of systemic lupus erythematosus (SLE) patent US8956607B2. 2015.
Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. 2016;167:419–32.e16.
Kenerson HL, Sullivan KM, Labadie KP, Pillarisetty VG, Yeung RS. Protocol for tissue slice cultures from human solid tumors to study therapeutic response. STAR Protoc. 2021;2:100574.
Jiang X, Seo YD, Sullivan KM, Pillarisetty VG. Establishment of slice cultures as a tool to study the cancer immune microenvironment. Methods Mol Biol. 2019;1884:283–95.
Jiang X, Seo YD, Chang JH, Coveler A, Nigjeh EN, Pan S, et al. Long-lived pancreatic ductal adenocarcinoma slice cultures enable precise study of the immune microenvironment. Oncoimmunology. 2017;6:e1333210.
Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34.
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765.
Mia S, Warnecke A, Zhang XM, Malmström V, Harris RA. An optimized protocol for human M2 macrophages using M‐CSF and IL‐4/IL‐10/TGF‐β yields a dominant immunosuppressive phenotype. Scand J Immunol. 2014;79:305–14.
Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–52.
Wu X, Roberto JB, Knupp A, Kenerson HL, Truong CD, Yuen SY, et al. Precision-cut human liver slice cultures as an immunological platform. J Immunol Methods. 2018;455:71–9.
Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, et al. Safety, antitumor activity, and immune activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. J Clin Oncol. 2016;34:3562–9.
Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–8.
Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–7.
Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–61.
Acknowledgements
We acknowledge Dr. James Oh Park, Raymond S. Yeung and Jonathan G. Sham and our patients for providing tumor specimens for this research. We acknowledge the NIH S10 grant (S10 OD016240) funding the Leica SP8X confocal microscope and Dr. Nathaniel Peters at the University of Washington W.M. Keck Microscopy Center for assistance with live image confocal microscopy.
Funding
VGP received funding from the US Army Medical Research Acquisition Activity (USAMRAA; CA150370P2), Seattle Translational Tumor Research, Brotman Baty Institute for Precision Medicine, and Merck Investigator Studies Program. TSK received funding from the UW/Fred Hutchinson Cancer Center Support Grant (CCSG), P30 CA015704, New Investigator Award. This work was also supported by the Washington Research Foundation, Stand Up to Cancer, and the National Institute of Neurological Disorders and Stroke, the National Institutes of Health (R25NS079200 to CIE), and the National Cancer Institute, National Institutes of Health (U54CA193461-03 and R01CA195718-02 to ECH).
Author information
Authors and Affiliations
Contributions
VGP and CAC conceived the idea, designed experiments, interpreted data, and provided funding and resources. KPL, SAK and KJB designed and performed experiments and analyzed and interpreted data. SKD, XJ, KMS, AFU and HLK assisted on slice culture experiments, collection and interpretation of data. SAK and KJB generated CAR T cells. KPL and VGP wrote the manuscript with input from SAK, KJB, KMS and CAC. All authors contributed feedback for the final manuscript.
Corresponding author
Ethics declarations
COMPETING INTERESTS
The authors declare that that they have read and understood the policy on declarations of interest. VGP is a member of the scientific advisory board for TriSalus Life Sciences. He served as a consultant for Merck & Company in 2018, GlaxoSmithKline in 2019, Imvax in 2019, Takeda in 2020, Umoja and Sensei in 2022. He has had research funding from AstraZeneca (completed), Ipsen (completed), Merck (completed), NGM (completed), and OncoResponse (current). The funders had no role in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. CAC is an inventor on issued patent US20170087185A1 “Genetic engineering of macrophages for immunotherapy”.
Ethical approval
All investigations were performed according to the principles expressed in the Declaration of Helsinki. Samples for slice culture were procured from patients undergoing hepatic or pancreatic resection who provided prior written-informed consent under research protocol (no. 1852) approved by the University of Washington Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labadie, K.P., Kreuser, S.A., Brempelis, K.J. et al. Production of an interleukin-10 blocking antibody by genetically engineered macrophages increases cancer cell death in human gastrointestinal tumor slice cultures. Cancer Gene Ther 30, 1227–1233 (2023). https://doi.org/10.1038/s41417-023-00632-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00632-z