Abstract
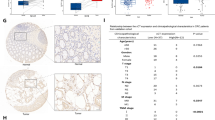
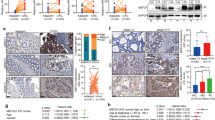
The molecular mechanism of network regulation in the occurrence and development of colorectal cancer (CRC) has been constantly improved. Here, we investigated the biological effects of TEAD4-MAD2L1 axis on proliferation and metastasis of human CRC cells. This study revealed that the expressions of MAD2L1 and TEAD4 in CRC tissues and CRC cell lines were significantly higher than those in adjacent epithelial tissues and normal intestinal epithelial cell line NCM460, and their expressions were significantly positively correlated; Moreover, inhibiting the expression of MAD2L1 or TEAD4 can inhibit the proliferation and migration of CRC cells and promote apoptosis. In addition, the promoter region of MAD2L1 gene has a TEAD4 binding site (motif sequence), and the transcription of MAD2L1 is positively regulated by TEAD4 protein; The inhibition of promotion/migration and promotion of apoptosis of CRC cells by silencing TEAD4 can be saved by the high expression of MAD2L1. In conclusion, our study suggests that the transcription and expression of MAD2L1 is regulated by TEAD4, which further promotes the proliferation and migration of CRC cells in vitro and in vivo, and inhibits apoptosis. MAD2L1 and TEAD4 are potential biomarkers for colorectal cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Kastrinos F, Samadder NJ, Burt RW. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 2020;158:389–403.
Harada S, Morlote D. Molecular pathology of colorectal cancer. Adv Anat Pathol. 2020;27:20–6.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23.
May KM, Hardwick KG. The spindle checkpoint. J Cell Sci. 2006;119:4139–42.
Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C). Q Rev Biophys. 2011;44:153–90.
Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us. Curr Opin Cell Biol. 2005;17:583–9.
Pfleger CM, Salic A, Lee E, Kirschner MW. Inhibition of Cdh1-APC by the MAD2-related protein MAD2L2: A novel mechanism for regulating Cdh1. Genes Dev. 2001;15:1759–64.
Bates M, Furlong F, Gallagher MF, Spillane CD, McCann A, O'Toole S, et al. Too MAD or not MAD enough: The duplicitous role of the spindle assembly checkpoint protein MAD2 in cancer. Cancer Lett. 2020;469:11–21.
Yu H. Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J Cell Biol. 2006;173:153–7.
Gibault F, Sturbaut M, Bailly F, Melnyk P, Cotelle P. Targeting Transcriptional Enhanced Associate Domains (TEADs). J Med Chem. 2018;61:5057–72.
Tomikawa J, Takada S, Okamura K, Terao M, Ogata-Kawata H, Akutsu H, et al. Exploring trophoblast-specific Tead4 enhancers through chromatin conformation capture assays followed by functional screening. Nucleic Acids Res. 2020;48:278–89.
He L, Yuan L, Sun Y, Wang P, Zhang H, Feng X, et al. Glucocorticoid Receptor Signaling Activates TEAD4 to Promote Breast Cancer Progression. Cancer Res. 2019;79:4399–411.
Figeac N, Mohamed AD, Sun C, Schonfelder M, Matallanas D, Garcia-Munoz A, et al. VGLL3 operates via TEAD1, TEAD3 and TEAD4 to influence myogenesis in skeletal muscle. J Cell Sci. 2019;132(13):jcs225946.
Wang L, Gao Y, Zhao X, Guo C, Wang X, Yang Y, et al. HOXD3 was negatively regulated by YY1 recruiting HDAC1 to suppress progression of hepatocellular carcinoma cells via ITGA2 pathway. Cell Prolif. 2020;53:e12835.
Li Q, Tong D, Guo C, Wu F, Li F, Wang X, et al. MicroRNA-145 suppresses gastric cancer progression by targeting Hu-antigen R. Am J Physiol Cell Physiol. 2020;318:C605–C14.
Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;174:1034–5.
Martinez-Jimenez F, Muinos F, Sentis I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, et al. A compendium of mutational cancer driver genes. Nat Rev Cancer. 2020;20:555–72.
Zhu XF, Yi M, He J, Tang W, Lu MY, Li T, et al. Pathological significance of MAD2L1 in breast cancer: An immunohistochemical study and meta analysis. Int J Clin Exp Pathol. 2017;10:9190–201.
Li J, He X, Wu X, Liu X, Huang Y, Gong Y. miR-139-5p inhibits lung adenocarcinoma cell proliferation, migration, and invasion by targeting MAD2L1. Comput Math Methods Med. 2020;2020:2953598.
Teixeira JH, Silva P, Faria J, Ferreira I, Duarte P, Delgado ML, et al. Clinicopathologic significance of BubR1 and Mad2 overexpression in oral cancer. Oral Dis. 2015;21:713–20.
Li Y, Bai W, Zhang J. MiR-200c-5p suppresses proliferation and metastasis of human hepatocellular carcinoma (HCC) via suppressing MAD2L1. Biomed Pharmacother.2017;92:1038–44.
Sun L, Li J, Yan B. Gene expression profiling analysis of osteosarcoma cell lines. Mol Med Rep. 2015;12:4266–72.
Wang Z, Katsaros D, Shen Y, Fu Y, Canuto EM, Benedetto C, et al. Biological and clinical significance of MAD2L1 and BUB1, genes frequently appearing in expression signatures for breast cancer prognosis. PLoS One. 2015;10:e0136246.
Wang Y, Wang F, He J, Du J, Zhang H, Shi H, et al. miR-30a-3p Targets MAD2L1 and regulates proliferation of gastric cancer cells. Onco Targets Ther. 2019;12:11313–24.
Chen M, Huang B, Zhu L, Chen K, Liu M, Zhong C. Structural and functional overview of TEAD4 in cancer biology. Onco Targets Ther. 2020;13:9865–74.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Zhang W, Li J, Wu Y, Ge H, Song Y, Wang D, et al. TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma. Cancer Cell Int. 2018;18:178.
Li J, Li Z, Wu Y, Wang Y, Wang D, Zhang W, et al. The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma. Cell Death Dis. 2019;10:603.
Acknowledgements
We thank the Key Laboratory of environment and genes related to diseases (Xi’an Jiaotong University) and the biomedical experimental center of Xi’an Jiaotong University for providing experimental sites and equipment. We also thank Mr. Xiaofei Wang from the large instrument-sharing center of Xi’an Jiaotong University for his help in flow analysis.
Funding
This work was financially supported by the Provincial Health Research Foundation in Shaanxi Province (2022E008), Projects of Xi’an Youth Talent Lift Program (095920221363), National Natural Science Foundation of China (81874192), the Natural Science Basic Research Plan in Shaanxi Province (2022JQ-980), the Key of Research and Development Plan in Shaanxi Province (2021SF-129).
Author information
Authors and Affiliations
Contributions
QL, CH, and MZ conceived and designed the experiments. QL, DT, XJ, FL, PM, and HW performed the experiments and analyzed the data. MC, QJ, and JZ performed bioinformatics analysis. QL, DT, and MZ wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Tong, D., Jing, X. et al. MAD2L1 is transcriptionally regulated by TEAD4 and promotes cell proliferation and migration in colorectal cancer. Cancer Gene Ther 30, 727–737 (2023). https://doi.org/10.1038/s41417-022-00586-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00586-8