Abstract
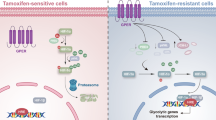
Chemotherapy can improve the prognosis and overall survival of breast cancer patients, but chemoresistance continues a major problem in clinical. Most breast cancer is estrogen receptor (ER) positive but responds less to neoadjuvant or adjuvant chemotherapy than ER-negative breast cancer. The Nogo-B receptor (NgBR) increases the chemoresistance of ER-positive breast cancer by facilitating oncogene signaling pathways. Here, we further investigated the potential role of NgBR as a novel target to overcome glycolysis-dependent paclitaxel resistance in ER-positive breast cancer. NgBR knockdown inhibited glycolysis and promoted paclitaxel-induced apoptosis by attenuating HIF-1α expression in ER-positive breast cancer cells via NgBR-mediated estrogen receptor alpha (ERα)/hypoxia-inducible factor-1 alpha (HIF-1α) and nuclear factor-kappa B subunit (NF-κB)/HIF-1α signaling pathways. A ChIP assay further confirmed that NgBR overexpression not only facilitates ERα binding to HIF-1α and GLUT1 genes but also promotes HIF-1α binding to GLUT1, HK2, and LDHA genes, which further promotes glycolysis and induces paclitaxel resistance. In conclusion, our study suggests that NgBR expression is essential for maintaining the metabolism and paclitaxel resistance of ER-positive breast cancer, and the NgBR can be a new therapeutic target for improving chemoresistance in ER-positive breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Please contact the corresponding authors for specific data and information.
Change history
15 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41417-022-00553-3
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin. 2021;71:7–33.
Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34:3617–26.
Society AC. Breast cancer facts & figures 2019–2020. Atlanta, GA, USA: American Cancer Society, Inc; 2019.
Waks AG, Winer EP. Breast cancer treatment: a review. J Am Med Assoc. 2019;321:288–300.
Jin Y, Hu W, Liu T, Rana U, Aguilera-Barrantes I, Kong A, et al. Nogo-B receptor increases the resistance of estrogen receptor positive breast cancer to paclitaxel. Cancer Lett. 2018;419:233–44.
McAndrew NP, Finn RS. Management of ER-positive metastatic breast cancer. Semin Oncol. 2020;47:270–7.
Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol. 2019;16:27–44.
Subramani R, Nandy SB, Pedroza DA, Lakshmanaswamy R. Role of growth hormone in breast cancer. Endocrinology. 2017;158:1543–55.
Varghese E, Samuel SM, Liskova A, Samec M, Kubatka P, Busselberg D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers. 2020;12:2252.
Sui M, Zhang H, Fan W. The role of estrogen and estrogen receptors in chemoresistance. Curr Medicinal Chem. 2011;18:4674–83.
Němcová-Fürstová V, Kopperová D, Balušíková K, Ehrlichová M, Brynychová V, Václavíková R, et al. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol Appl Pharmacol. 2016;310:215–28.
Murray S, Briasoulis E, Linardou H, Bafaloukos D, Papadimitriou C. Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat Rev. 2012;38:890–903.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11.
Doktorova H, Hrabeta J, Khalil MA, Eckschlager T. Hypoxia-induced chemoresistance in cancer cells: the role of not only HIF-1. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:166–77.
Wouters BG, van den Beucken T, Magagnin MG, Lambin P, Koumenis C. Targeting hypoxia tolerance in cancer. Drug Resist Updat. 2004;7:25–40.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30.
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Therapeut. 2012;11:1672–82.
Barbosa AM, Martel F. Targeting glucose transporters for breast cancer therapy: the effect of natural and synthetic compounds. Cancers. 2020;12:154.
Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–28.
Wang J, Tao M, Wang T, Wang Z, Xiao J, Ding S, et al. [Knockdown of hexokinase 2 (HK2) inhibits breast cancer cell proliferation and reduces their resistance to fluorouracil]. Xi bao yu fen zi mian yi xue za zhi = Chin J Cell Mol Immunol. 2021;37:722–7.
Das CK, Parekh A, Parida PK, Bhutia SK, Mandal M. Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim Biophys Acta Mol Cell Res. 2019;1866:1004–18.
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33.
Lu Y, Wang L, Ding W, Wang D, Wang X, Luo Q, et al. Ammonia mediates mitochondrial uncoupling and promotes glycolysis via HIF-1 activation in human breast cancer MDA-MB-231cells. Biochem Biophys Res Commun. 2019;519:153–9.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, et al. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha. Cancer Res. 2016;76:2231–42.
George AL, Rajoria S, Suriano R, Mittleman A, Tiwari RK. Hypoxia and estrogen are functionally equivalent in breast cancer-endothelial cell interdependence. Mol Cancer. 2012;11:80.
Li YK, Xie YJ, Wu DC, Long SL, Tang S, Mo ZC. NogoB receptor in relevant carcinoma: current achievements, challenges and aims (review). Int J Oncol. 2018;53:1827–35.
Zhao B, Xu B, Hu W, Song C, Wang F, Liu Z, et al. Comprehensive proteome quantification reveals NgBR as a new regulator for epithelial-mesenchymal transition of breast tumor cells. J Proteom. 2015;112:38–52.
Zhao B, Hu W, Kumar S, Gonyo P, Rana U, Liu Z, et al. The Nogo-B receptor promotes Ras plasma membrane localization and activation. Oncogene. 2017;36:3406–16.
Pula B, Olbromski M, Owczarek T, Ambicka A, Witkiewicz W, Ugorski M, et al. Nogo-B receptor expression correlates negatively with malignancy grade and ki-67 antigen expression in invasive ductal breast carcinoma. Anticancer Res. 2014;34:4819–28.
Yang J, AlTahan A, Jones DT, Buffa FM, Bridges E, Interiano RB, et al. Estrogen receptor-alpha directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer. Proc Natl Acad Sci USA 2015;112:15172–7.
Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, et al. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci USA 2006;103:10997–1002.
Zhao B, Chun C, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, et al. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood 2010;116:5423–33.
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510.
Zang H, Li Y, Zhang X, Huang G. Circ-RNF111 contributes to paclitaxel resistance in breast cancer by elevating E2F3 expression via miR-140-5p. Thorac Cancer. 2020;11:1891–903.
Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820–31.
Cao L, Wang M, Dong Y, Xu B, Chen J, Ding Y, et al. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020;11:145.
Jiang H, Jia D, Zhang B, Yang W, Dong Z, Sun X, et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res Cardiol. 2020;115:28.
Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78.
Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532.
Groheux D, Majdoub M, Sanna A, de Cremoux P, Hindie E, Giacchetti S, et al. Early metabolic response to neoadjuvant treatment: FDG PET/CT criteria according to breast cancer subtype. Radiology. 2015;277:358–71.
Cong Y, Cui Y, Zhu S, Cao J, Zou H, Martin TA, et al. Tim-3 promotes cell aggressiveness and paclitaxel resistance through NF-κB/STAT3 signalling pathway in breast cancer cells. Chin J Cancer Res = Chung-kuo yen cheng yen chiu. 2020;32:564–79.
Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y, et al. Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance. Cell Death Differ. 2022;29:381–92.
Li H, Fu L, Liu B, Lin X, Dong Q, Wang E. Ajuba overexpression regulates mitochondrial potential and glucose uptake through YAP/Bcl-xL/GLUT1 in human gastric cancer. Gene. 2019;693:16–24.
Wu Q, Ba-Alawi W, Deblois G, Cruickshank J, Duan S, Lima-Fernandes E, et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat Commun. 2020;11:4205.
Zhang T, Zhu X, Wu H, Jiang K, Zhao G, Shaukat A, et al. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: combined administration of polydatin and 2-deoxy-D-glucose. J Cell Mol Med. 2019;23:3711–23.
Zhao T, Jin F, Xiao D, Wang H, Huang C, Wang X, et al. IL-37/ STAT3/ HIF-1α negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics. 2020;10:4088–100.
Jin J, Qiu S, Wang P, Liang X, Huang F, Wu H, et al. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res: CR. 2019;38:377.
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510.
Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM, et al. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun. 2018;9:4456.
Zhang T, Guo S, Zhu X, Qiu J, Deng G, Qiu C. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J Cell Mol Med. 2020;24:8430–40.
Gao X, Wu Y, Qiao L, Feng X. SENP2 suppresses NF-κB activation and sensitizes breast cancer cells to doxorubicin. Eur J Pharmacol. 2019;854:179–86.
Liang S, Chen Z, Jiang G, Zhou Y, Liu Q, Su Q, et al. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-κB/IL-6 signals. Cancer Lett. 2017;386:12–23.
Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–44.
Yang J, Jubb AM, Pike L, Buffa FM, Turley H, Baban D, et al. The histone demethylase JMJD2B is regulated by estrogen receptor alpha and hypoxia, and is a key mediator of estrogen induced growth. Cancer Res. 2010;70:6456–66.
Yang J, AlTahan A, Jones DT, Buffa FM, Bridges E, Interiano RB, et al. Estrogen receptor-α directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer. Proc Natl Acad Sci USA 2015;112:15172–7.
Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol. 1988;6:1076–87.
Smart E, Semina SE, Frasor J. Update on the role of NFκB in promoting aggressive phenotypes of estrogen receptor-positive breast cancer. Endocrinology. 2020;161:bqaa152.
Fumagalli D, Wilson TR, Salgado R, Lu X, Yu J, O’Brien C, et al. Somatic mutation, copy number and transcriptomic profiles of primary and matched metastatic estrogen receptor-positive breast cancers. Ann Oncol. 2016;27:1860–6.
Burstein HJ. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. New Engl J Med. 2020;383:2557–70.
Acknowledgements
Thanks to the following projects for supporting this study: National Natural Science Foundation of China (No. 82003173), Jilin Province Department of Finance (JLSCZD2019-030), Health Commission of Jilin Province (No. 2020Q016).
Author information
Authors and Affiliations
Contributions
CL: conceptualization, data curation, formal analysis, investigation, writing—original draft and writing—review & editing; SL: supervision and methodology; XZ: data curation and validation; CJ: resources and supervision; BZ: resources and supervision; LL: methodology and validation; QRM: conceptualization, supervision, and writing—review and editing; YJ: conceptualization, funding acquisition, resources, supervision and writing—review and editing; ZF: conceptualization, funding acquisition, resources, and writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All patients signed informed consent for this study, and their samples were anonymous. The research has been allowed by the Ethics Committee of the First Hospital of Jilin University (2020-066).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Li, S., Zhang, X. et al. Nogo-B receptor increases glycolysis and the paclitaxel resistance of estrogen receptor-positive breast cancer via the HIF-1α-dependent pathway. Cancer Gene Ther 30, 647–658 (2023). https://doi.org/10.1038/s41417-022-00542-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00542-6