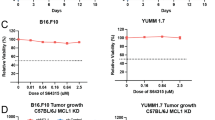
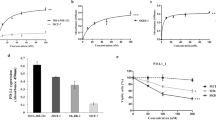
Abstract
Inhibitory receptors (IRs), such as the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), are cell surface molecules expressed on both normal epithelial, endothelial, and hematopoietic cells and on neoplastic cells. IRs are usually used by cancer cells to inhibit immune cell functions. Thus, CEACAM1 positive tumor cells can interact homophilically with CEACAM1 expressed on T and NK cells to inhibit their antibody-dependent cell-mediated cytotoxicity (ADCC). In this study, we investigated the effect of agonistic/activating anti-CEACAM1 monoclonal antibody (mAb) on melanoma cell lines in vitro and in vivo, following our hypothesis that activation of CEACAM1 on melanoma cells by distinct mAbs may induce inhibition of cancer cell proliferation and/or their death. To address this, we established an activating anti-CEACAM1 mAb (CCM5.01) and characterized its binding to the CEACAM1 receptor. Using this mAb, we assessed the expression of CEACAM1 on four different human melanoma cell lines by western blot and flow cytometry and determined its effect on cell viability in vitro by MTT assay. Furthermore, we evaluated the mAb mechanism of action and found that binding of CEACAM1 with CCM5.01 induced SHP1 phosphorylation and p53 activation resulting in melanoma cell apoptosis. For in vivo studies, a xenograft model of melanoma was performed by injection of Mel-14 cells subcutaneously (s.c.) in SCID/Beige mice followed by intraperitoneal (i.p.) injection of CCM5.01 or of IgG1 isotype control every other day. CCM5.01 treated mice showed a slight but not significant decrease in tumor weight in comparison to the control group. Based on the obtained data, we suggest that activating CEACAM1 on melanoma cells might be a promising novel approach to fight cancers expressing this IR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on request.
Change history
03 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41417-023-00594-2
References
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801.
Markel G, Sapir Y, Mandel I, Hakim M, Shaked R, Meilin E, et al. Inhibition of the novel immune checkpoint CEACAM1 to enhance anti-tumor immunological activity. J Clin Oncol. 2016;34:3044.
Helfrich I, Singer BB. Size matters: the functional role of the CEACAM1 isoform signature and its impact for NK cell-mediated killing in melanoma. Cancers. 2019;11:356.
Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Öbrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168:5139–46.
Öbrink B. On the role of CEACAM1 in cancer. Lung Cancer. 2008;60:309–12.
Klaile E, Müller MM, Schäfer MR, Clauder AK, Feer S, Heyl KA, et al. Binding of Candida albicans to human CEACAM1 and CEACAM6 modulates the inflammatory response of intestinal epithelial cells. MBio. 2017;8e02142.
Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche CI, et al. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol. 2017;2:16189.
Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, N’Guessan PD, et al. CEACAM1 inhibits Toll-like receptor 2–triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol. 2008;9:1270–8.
Sheikh A, Tumala B, Vickers TJ, Alvarado D, Ciorba MA, Bhuiyan TR, et al. CEACAMs serve as toxin-stimulated receptors for enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA. 2020;117:29055–62.
van Sorge NM, Bonsor DA, Deng L, Lindahl E, Schmitt V, Lyndin M, et al. Bacterial protein domains with a novel Ig‐like fold target human CEACAM receptors. EMBO J. 2021;40:e106103.
Ambrosi C, Scribano D, Sarshar M, Zagaglia C, Singer BB, Palamara AT. Acinetobacter baumannii targets human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) for invasion of pneumocytes. mSystems. 2020;5:e00604-20.
Gur C, Maalouf N, Shhadeh A, Berhani O, Singer BB, Bachrach G, et al. Fusobacterium nucleatum supresses anti-tumor immunity by activating CEACAM1. Oncoimmunology. 2019;8:e1581531.
Galaski J, Shhadeh A, Umaña A, Yoo CC, Arpinati L, Isaacson B, et al. Fusobacterium nucleatum CbpF mediates inhibition of T cell function through CEACAM1 activation. Front Cell Infect Microbiol. 2021;11:692544.
Miura HS, Nakagaki K, Taguchi F. N-terminal domain of the murine coronavirus receptor CEACAM1 is responsible for fusogenic activation and conformational changes of the spike protein. J Virol. 2004;78:216–23.
Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–10.
Ortenberg R, Sapir Y, Raz L, Hershkovitz L, Ben Arav A, Sapoznik S, et al. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Mol Cancer Ther. 2012;11:1300–10.
Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, et al. Expression of CEACAM1 in adenocarcinoma of the lung: a factor of independent prognostic significance. J Clin Oncol. 2002;20:4279–84.
Takeuchi A, Yokoyama S, Nakamori M, Nakamura M, Ojima T, Yamaguchi S, et al. Loss of CEACAM1 is associated with poor prognosis and peritoneal dissemination of patients with gastric cancer. Sci Rep. 2019;9:12702.
Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, et al. Neutrophil extracellular trap–associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol. 2020;204:2285–94.
Pan H, Shively JE. Carcinoembryonic antigen-related cell adhesion molecule-1 regulates granulopoiesis by inhibition of granulocyte colony-stimulating factor receptor. Immunity. 2010;33:620–31.
Landolina N, Zaffran I, Smiljkovic D, Serrano-Candelas E, Schmiedel D, Friedman S, et al. Activation of Siglec-7 results in inhibition of in vitro and in vivo growth of human mast cell leukemia cells. Pharm Res. 2020;158:104682.
Turner MA, Amirfakhri S, Nishino H, Lwin TM, Savides TJ, Reid TR, et al. A patient-derived orthotopic xenograft model of gastroesophageal-junction adenocarcinoma translated to the clinic by tumor-targeting fluorescent antibodies to carcinoembryonic-antigen-related cell-adhesion molecules. In Vivo 2021;35:1959–63.
Hollandsworth HM, Amirfakhri S, Filemoni F, Schmitt V, Wennemuth G, Schmidt A, et al. Anti-carcinoembryonic antigen-related cell adhesion molecule antibody for fluorescence visualization of primary colon cancer and metastases in patient-derived orthotopic xenograft mouse models. Oncotarget. 2020;11:429–39.
Legrand F, Landolina N, Zaffran I, Emeh RO, Chen E, Klion AD, et al. Siglec-7 on peripheral blood eosinophils: Surface expression and function. Allergy. 2019;74:1257–65.
Ravindranath MH, Shen P, Habal N, Soh D, Nishimoto K, Gonzales A, et al. Does human melanoma express carcinoembryonic antigen? Anticancer Res. 2000;20:3083–92.
Sappino AP, Buser R, Seguin Q, Fernet M, Lesne L, Gumy-Pause F, et al. The CEACAM1 tumor suppressor is an ATM and p53-regulated gene required for the induction of cellular senescence by DNA damage. Oncogenesis. 2012;1:e7.
Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247–50.
Ieda J, Yokoyama S, Tamura K, Takifuji K, Hotta T, Matsuda K, et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int J Cancer. 2011;129:1351–61.
Kiriyama S, Yokoyama S, Ueno M, Hayami S, Ieda J, Yamamoto N, et al. CEACAM1 long cytoplasmic domain isoform is associated with invasion and recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2014;21:505–14.
Ortenberg R, Galore-Haskel G, Greenberg I, Zamlin B, Sapoznik S, Greenberg E, et al. CEACAM1 promotes melanoma cell growth through Sox-2. Neoplasia. 2014;16:451–60.
Wicklein D, Otto B, Suling A, Elies E, Lüers G, Lange T, et al. CEACAM1 promotes melanoma metastasis and is involved in the regulation of the EMT associated gene network in melanoma cells. Sci Rep. 2018;8:11893.
Sapoznik S, Ortenberg R, Schachter J, Markel G. CEACAM1 in malignant melanoma: a diagnostic and therapeutic target. Curr Top Med Chem. 2012;12:3–10.
Xu J, Liu B, Ma S, Zhang J, Ji Y, Xu L, et al. Characterizing the tumor suppressor role of CEACAM1 in multiple myeloma. Cell Physiol Biochem. 2018;45:1631–40.
Watt SM. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood. 2001;98:1469–79.
Tchoupa AK, Lichtenegger S, Reidl J, Hauck CR. Outer membrane protein P1 is the CEACAM-binding adhesin of Haemophilus influenzae. Mol Microbiol. 2015;98:440–55.
Nagaishi T, Pao L, Lin S-H, Iijima H, Kaser A, Qiao S-W, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–81.
Singer BB, Scheffrahn I, Obrink B. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 2000;60:1236–44.
Singer BB, Scheffrahn I, Kammerer R, Suttorp N, Ergun S, Slevogt H. Deregulation of the CEACAM expression pattern causes undifferentiated cell growth in human lung adenocarcinoma cells. PLoS ONE. 2010;5:e8747.
Ueshima C, Kataoka TR, Takei Y, Hirata M, Sugimoto A, Hirokawa M, et al. CEACAM1 long isoform has opposite effects on the growth of human mastocytosis and medullary thyroid carcinoma cells. Cancer Med. 2017;6:845–56.
Chen Z, Chen L, Qiao S-W, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–93.
Bachelet I, Munitz A, Berent-Maoz B, Mankuta D, Levi-Schaffer F. Suppression of normal and malignant kit signaling by a bispecific antibody linking kit with CD300a. J Immunol. 2008;180:6064–9.
Patel PC, Lee HSW, Ming AYK, Rath A, Deber CM, Yip CM, et al. Inside-out signaling promotes dynamic changes in the carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1) oligomeric state to control its cell adhesion properties. J Biol Chem. 2013;288:29654–69.
Kim WM, Huang Y-H, Gandhi A, Blumberg RS. CEACAM1 structure and function in immunity and its therapeutic implications. Semin Immunol. 2019;42:101296.
Acknowledgements
The authors thank Ofra Moshel, PhD (The Core Research Facility, The Institute of Drug Research, The Hebrew University of Jerusalem) who provided advice and technical help for FC and MST experiments and analyses.
Funding
This work was partially funded by grants from Aimwell Charitable Trust (UK), INTEGRA holdings, the Israel Cancer Association (No. 20210024) to FL-S, and the Israeli Scholarship Education Foundation (ISEF) to IZ.
Author information
Authors and Affiliations
Contributions
Designing research studies: FL-S. Conducting experiments: IZ, NL, PG, TLR. Acquiring data: IZ, NL, TLR, SJ, BBS. Analyzing data: IZ, NL, PG, TLR, SJ, BBS, FL-S. Providing reagents: OM, SJ, BBS, FL-S. Writing the manuscript: IZ, FL-S, BBS. Revising the manuscript: IZ, BBS, FL-S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have read and consented to the manuscript’s publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaffran, I., Landolina, N., Gaur, P. et al. Activation of CEACAM1 with an agonistic monoclonal antibody results in inhibition of melanoma cells. Cancer Gene Ther 29, 1676–1685 (2022). https://doi.org/10.1038/s41417-022-00486-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00486-x