Abstract
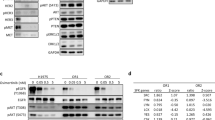
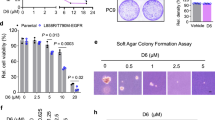
SHP2, a protein tyrosine phosphatase, plays a critical role in fully activating oncogenic signaling pathways such as Ras/MAPK downstream of cell surface tyrosine receptors (e.g., EGFR), which are often activated in human cancers, and thus has emerged as an attractive cancer therapeutic target. This study focused on evaluating the therapeutic potential of the novel SHP2 degrader, SHP2-D26 (D26), either alone or in combination, against non-small cell lung cancer (NSCLC) cells. While all tested NSCLC cell lines responded to D26 with IC50s of < 8 μM, a few cell lines (4/14) were much more sensitive than others with IC50s of ≤ 4 μM. There was no clear association between basal levels of SHP2 and cell sensitivities to D26. Moreover, D26 rapidly and potently decreased SHP2 levels in different NSCLC cell lines in a sustained way regardless of cell sensitivities to D26, suggesting that additional factors may impact cell response to D26. We noted that suppression of p70S6K/S6, but not ERK1/2, was associated with cell responses to D26. In the sensitive cell lines, D26 effectively increased Bim levels while decreasing Mcl-1 levels accompanied with the induction of apoptosis. When combined with the third generation EGFR inhibitor, osimertinib (AZD9291), synergistic effects on decreasing the survival of different osimertinib-resistant cell lines were observed with enhanced induction of apoptosis. Although D26 alone exerted moderate inhibition of the growth of NSCLC xenografts, the combination of osimertinib and D26 effectively inhibited the growth of osimertinib-resistant xenografts, suggesting promising efficacy in overcoming acquired resistance to osimertinib.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data presented have been included in this report.
Change history
10 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41417-022-00479-w
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin. 2022;72:7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49.
Tang K, Jia YN, Yu B, Liu HM. Medicinal chemistry strategies for the development of protein tyrosine phosphatase SHP2 inhibitors and PROTAC degraders. Eur J Med Chem. 2020;204:112657.
Song Y, Zhao M, Zhang H, Yu B. Double-edged roles of protein tyrosine phosphatase SHP2 in cancer and its inhibitors in clinical trials. Pharmacol Ther 2021:107966.
Yuan X, Bu H, Zhou J, Yang CY, Zhang H. Recent advances of SHP2 inhibitors in cancer therapy: current development and clinical application. J Med Chem. 2020;63:11368–96.
Wang M, Lu J, Wang M, Yang CY, Wang S. Discovery of SHP2-D26 as a first, potent, and effective PROTAC degrader of SHP2 protein. J Med Chem. 2020;63:7510–28.
Vemulapalli V, Donovan KA, Seegar TCM, Rogers JM, Bae M, Lumpkin RJ, et al. Targeted degradation of the oncogenic phosphatase SHP2. Biochemistry. 2021;60:2593–609.
Zheng M, Liu Y, Wu C, Yang K, Wang Q, Zhou Y, et al. Novel PROTACs for degradation of SHP2 protein. Bioorg Chem. 2021;110:104788.
He L, Li Y, Huang X, Cheng H, Ke Y, Wang L. The prognostic significance of SHP2 and its binding protein Hook1 in non-small cell lung cancer. OncoTargets Ther. 2019;12:5897–906.
Karachaliou N, Cardona AF, Bracht JWP, Aldeguer E, Drozdowskyj A, Fernandez-Bruno M, et al. Integrin-linked kinase (ILK) and src homology 2 domain-containing phosphatase 2 (SHP2): Novel targets in EGFR-mutation positive non-small cell lung cancer (NSCLC). EBioMedicine. 2019;39:207–14.
Chen MJ, Wang YC, Wu DW, Chen CY, Lee H. Association of nuclear localization of SHP2 and YAP1 with unfavorable prognosis in non-small cell lung cancer. Pathol Res Pr. 2019;215:801–6.
Mainardi S, Mulero-Sanchez A, Prahallad A, Germano G, Bosma A, Krimpenfort P, et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. 2018;24:961–7.
Ruess DA, Heynen GJ, Ciecielski KJ, Ai J, Berninger A, Kabacaoglu D, et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat Med. 2018;24:954–60.
Ito M, Codony-Servat J, Gimenez-Capitan A, Serra-Mitjans M, Perez-Ochoa F, Llige D, et al. Src-Homology 2 Domain-Containing Phosphatase 2 in Resected EGFR mutation-positive lung adenocarcinoma. JTO Clin Res Rep. 2020;1:100084.
Schneeberger VE, Ren Y, Luetteke N, Huang Q, Chen L, Lawrence HR, et al. Inhibition of Shp2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget. 2015;6:6191–202.
Kano H, Ichihara E, Watanabe H, Nishii K, Ando C, Nakasuka T, et al. SHP2 inhibition enhances the effects of tyrosine kinase inhibitors in preclinical models of treatment-naive ALK-, ROS1-, or EGFR-altered non-small cell lung cancer. Mol Cancer Ther. 2021;20:1653–62.
Pudelko L, Jaehrling F, Reusch C, Vitri S, Stroh C, Linde N, et al. SHP2 inhibition influences therapeutic response to tepotinib in tumors with MET alterations. iScience. 2020;23:101832.
Shi P, Oh YT, Deng L, Zhang G, Qian G, Zhang S, et al. Overcoming acquired resistance to AZD9291, a third-generation EGFR Inhibitor, through modulation of MEK/ERK-dependent Bim and Mcl-1 degradation. Clin Cancer Res. 2017;23:6567–79.
Koo J, Yue P, Gal AA, Khuri FR, Sun SY. Maintaining glycogen synthase kinase-3 activity is critical for mTOR kinase inhibitors to inhibit cancer cell growth. Cancer Res. 2014;74:2555–68.
Ren H, Chen M, Yue P, Tao H, Owonikoko TK, Ramalingam SS, et al. The combination of RAD001 and NVP-BKM120 synergistically inhibits the growth of lung cancer in vitro and in vivo. Cancer Lett. 2012;325:139–46.
Zong D, Gu J, Cavalcante GC, Yao W, Zhang G, Wang S, et al. BRD4 levels determine the response of human lung cancer cells to BET degraders that potently induce apoptosis through suppression of Mcl-1. Cancer Res. 2020;80:2380–93.
Ren H, Koo J, Guan B, Yue P, Deng X, Chen M, et al. The E3 ubiquitin ligases beta-TrCP and FBXW7 cooperatively mediates GSK3-dependent Mcl-1 degradation induced by the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer. 2013;12:146.
Qian G, Yao W, Zhang S, Bajpai R, Hall WD, Shanmugam M, et al. Co-inhibition of BET and proteasome enhances ER stress and Bim-dependent apoptosis with augmented cancer therapeutic efficacy. Cancer Lett. 2018;435:44–54.
Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9.
Shi P, Oh YT, Zhang G, Yao W, Yue P, Li Y, et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett. 2016;380:494–504.
Hasenjager A, Gillissen B, Muller A, Normand G, Hemmati PG, Schuler M, et al. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene. 2004;23:4523–35.
Sun SY, Yue P, Shroot B, Hong WK, Lotan R. Induction of apoptosis in human non-small cell lung carcinoma cells by the novel synthetic retinoid CD437. J Cell Physiol. 1997;173:279–84.
Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, et al. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–65.
Li Y, Zang H, Qian G, Owonikoko TK, Ramalingam SR, Sun SY. ERK inhibition effectively overcomes acquired resistance of epidermal growth factor receptor-mutant non-small cell lung cancer cells to osimertinib. Cancer. 2020;126:1339–50.
Sun Y, Meyers BA, Czako B, Leonard P, Mseeh F, Harris AL, et al. Allosteric SHP2 Inhibitor, IACS-13909, overcomes EGFR-dependent and EGFR-independent resistance mechanisms toward Osimertinib. Cancer Res. 2020;80:4840–53.
Xia L, Yang F, Wu X, Li S, Kan C, Zheng H, et al. SHP2 inhibition enhances the anticancer effect of Osimertinib in EGFR T790M mutant lung adenocarcinoma by blocking CXCL8 loop mediated stemness. Cancer Cell Int. 2021;21:337.
Zito CI, Qin H, Blenis J, Bennett AM. SHP-2 regulates cell growth by controlling the mTOR/S6 kinase 1 pathway. J Biol Chem. 2007;282:6946–53.
Mercan F, Lee H, Kolli S, Bennett AM. Novel role for SHP-2 in nutrient-responsive control of S6 kinase 1 signaling. Mol Cell Biol. 2013;33:293–306.
Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell. 2006;10:317–27.
Sharma N, Kumar V, Everingham S, Mali RS, Kapur R, Zeng LF, et al. SH2 domain-containing phosphatase 2 is a critical regulator of connective tissue mast cell survival and homeostasis in mice. Mol Cell Biol. 2012;32:2653–63.
Selimoglu-Buet D, Gallais I, Denis N, Guillouf C, Moreau-Gachelin F. Oncogenic kit triggers Shp2/Erk1/2 pathway to down-regulate the pro-apoptotic protein Bim and to promote apoptosis resistance in leukemic cells. PLoS One. 2012;7:e49052.
Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19:2075–83.
Dardaei L, Wang HQ, Singh M, Fordjour P, Shaw KX, Yoda S, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24:512–7.
Acknowledgements
We thank Dr. A. Hammond in our department for editing the manuscript. SSR and S-YS are Georgia Research Alliance Distinguished Cancer Scientists. This study was supported by Emory University Winship Cancer Institute lung cancer pilot fund (to S-YS).
Author information
Authors and Affiliations
Contributions
Conceptualization: YD, SW, SSR, and SYS. Investigation: YD, GM, KAV, DW, MW, and CW. Methodology: YD, GM, KAV, DW, MW, CW and SYS. Writing/Original Draft: YD and SYS. Review/Editing: GM, KAV, DW, MW, CW, SW, and SSR. Funding Acquisition: SSR and SYS. Supervision: SW and SYS
Corresponding author
Ethics declarations
Competing interests
SSR is on consulting/advisory board for AstraZeneca, BMS, Merck, Roche, Tesaro and Amgen. The University of Michigan has filed a patent application on SHP2-D26 and its analogs. SW and MW are co-inventors of the patent application, which has been licensed by Roivant Sciences and Proteovant Therapeutics Inc. SW is a paid consultant to Roivant Sciences and Proteovant Therapeutics. The University of Michigan has received a research contract for which SW serves as the principal investigator. Other authors disclose that they have no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Deng, Y., Ma, G., Vallega, K.A. et al. Therapeutic efficacy of the novel SHP2 degrader SHP2-D26, alone or in combination, against lung cancer is associated with modulation of p70S6K/S6, Bim and Mcl-1. Cancer Gene Ther 29, 1558–1569 (2022). https://doi.org/10.1038/s41417-022-00472-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00472-3