Abstract
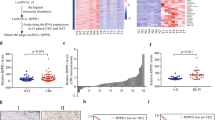
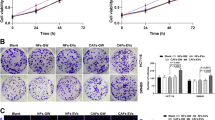
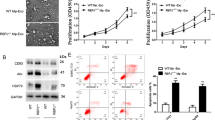
Colorectal cancer (CRC) is a prevalent malignancy with high incidence and low 5-year survival. Long non-coding RNAs (lncRNAs), a kind of specific RNA transcript, are increasingly implicated in tumor growth, metastasis, invasion, and prognosis by regulating the tumor microenvironment in extracellular vesicles (EVs). This study aims at investigating the potential effect of lncRNA HLA-F-AS1 on CRC by affecting the profilin 1 (PFN1) expression pattern in the tumor EVs. The expression patterns of HLA-F-AS1 and miR-375 were determined by RT-qPCR in the CRC tissues and cells. CCK-8 and Transwell assays were conducted to detect the cell proliferation and migration, and invasion, respectively. Western blot analysis was performed to measure the expression pattern of the epithelial–mesenchymal transition (EMT) markers. Bioinformatics prediction website and dual-luciferase reporter assay were conducted to verify the interaction between HLA-F-AS1 and miR-375. The CRC-derived EVs were extracted with the expression pattern of PFN1 determined by ELISA, while its effect on the macrophage polarization was assessed by flow cytometry. The effect of PFN1-treated macrophages on CRC cell proliferation and migration was observed by subcutaneous tumorigenesis experiments in nude mice. The results indicated that the HLA-F-AS1 expression pattern was increased in the CRC tissues and cells, which promoted the migration, invasion, and EMT of CRC cells in vitro. Mechanistically, HLA-F-AS1 competitively bound to miR-375 and inversely regulated miR-375 expression pattern. Interestingly, PFN1 was identified as a direct target of miR-375, and positively modulated by HLA-F-AS1 by binding to miR-375. Overexpression of HLA-F-AS1 repressed miR-375 and promoted the PFN1 expression pattern in CRC cells and CRC-derived EVs, further promoting M2 polarization of macrophages. Furthermore, macrophages treated with PFN1 in CRC-derived EVs stimulated CRC cell proliferation and migration in vitro and in vivo. Collectively, these outcomes highlight that HLA-F-AS1 promotes the expression pattern of PFN1 in CRC-EVs by inhibiting miR-375, thereby polarizing macrophages toward M2 phenotype, and aggravating the tumorigenesis of CRC, eliciting that HLA-F-AS1 may serve as a viable and promising therapeutic strategy for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet 2019;394:1467–80.
Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109–31.
Buccafusca G, Proserpio I, Tralongo AC, Rametta Giuliano S, Tralongo P. Early colorectal cancer: diagnosis, treatment and survivorship care. Crit Rev Oncol Hematol. 2019;136:20–30.
Sun Z, Liu J, Chen C, Zhou Q, Yang S, Wang G, et al. The biological effect and clinical application of long noncoding RNAs in colorectal cancer. Cell Physiol Biochem. 2018;46:431–41.
Kim T, Croce CM. Long noncoding RNAs: undeciphered cellular codes encrypting keys of colorectal cancer pathogenesis. Cancer Lett. 2018;417:89–95.
Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20:5758.
Liu ML, Zhang Q, Yuan X, Jin L, Wang LL, Fang TT, et al. Long noncoding RNA RP4 functions as a competing endogenous RNA through miR-7-5p sponge activity in colorectal cancer. World J Gastroenterol. 2018;24:1004–12.
Shang AQ, Wang WW, Yang YB, Gu CZ, Ji P, Chen C, et al. Knockdown of long noncoding RNA PVT1 suppresses cell proliferation and invasion of colorectal cancer via upregulation of microRNA-214-3p. Am J Physiol Gastrointest Liver Physiol. 2019;317:G222–G32.
Huang Y, Sun H, Ma X, Zeng Y, Pan Y, Yu D, et al. HLA-F-AS1/miR-330-3p/PFN1 axis promotes colorectal cancer progression. Life Sci. 2019;254:117180.
Xu X, Chen X, Xu M, Liu X, Pan B, Qin J, et al. miR-375-3p suppresses tumorigenesis and partially reverses chemoresistance by targeting YAP1 and SP1 in colorectal cancer cells. Aging. 2019;11:7357–85.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21.
Ruiz-Lopez L, Blancas I, Garrido JM, Mut-Salud N, Moya-Jodar M, Osuna A, et al. The role of exosomes on colorectal cancer: a review. J Gastroenterol Hepatol. 2018;33:792–9.
Wu J, Li H, Xie H, Wu X, Lan P. The malignant role of exosomes in the communication among colorectal cancer cell, macrophage and microbiome. Carcinogenesis. 2019;40:601–10.
Wang M, Su Z, Amoah Barnie P. Crosstalk among colon cancer-derived exosomes, fibroblast-derived exosomes, and macrophage phenotypes in colon cancer metastasis. Int Immunopharmacol. 2020;81:106298.
Braster R, Bogels M, Beelen RH, van Egmond M. The delicate balance of macrophages in colorectal cancer; their role in tumour development and therapeutic potential. Immunobiology. 2017;222:21–30.
Zhong X, Chen B, Yang Z. The role of tumor-associated macrophages in colorectal carcinoma progression. Cell Physiol Biochem. 2018;45:356–65.
Romeo GR, Moulton KS, Kazlauskas A. Attenuated expression of profilin-1 confers protection from atherosclerosis in the LDL receptor null mouse. Circ Res. 2007;101:357–67.
Artyomov MN, Sergushichev A, Schilling JD. Integrating immunometabolism and macrophage diversity. Semin Immunol. 2016;28:417–24.
Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–95.
Ronnekleiv-Kelly SM, Burkhart RA, Pawlik TM. Molecular markers of prognosis and therapeutic targets in metastatic colorectal cancer. Surg Oncol. 2016;25:190–9.
Ding D, Han S, Zhang H, He Y, Li Y. Predictive biomarkers of colorectal cancer. Comput Biol Chem. 2019;83:107106.
ME IJ, Sanz-Pamplona R, Hermitte F, de Miranda N. Colorectal cancer: a paradigmatic model for cancer immunology and immunotherapy. Mol Asp Med. 2019;69:123–9.
Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34:13–25.
Wang C, Luo J, Chen Z, Ye M, Hong Y, Liu J, et al. MiR-375 impairs the invasive capabilities of hepatoma cells by targeting HIF1alpha under hypoxia. Dig Dis Sci. 2020. https://doi.org/10.1007/s10620-020-06202-9. Epub ahead of print.
Xu F, Ye ML, Zhang YP, Li WJ, Li MT, Wang HZ, et al. MicroRNA-375-3p enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Cancer Sci. 2020;111:1528–41.
Zhang Y, Mou C, Shang M, Jiang M, Xu C. Long noncoding RNA RP11-626G11.3 promotes the progression of glioma through miR-375-SP1 axis. Mol Carcinog. 2020;59:492–502.
Mazzatti DJ, Pawelec G, Longdin R, Powell JR, Forsey RJ. SELDI-TOF-MS ProteinChip array profiling of T-cell clones propagated in long-term culture identifies human profilin-1 as a potential bio-marker of immunosenescence. Proteome Sci. 2007;5:7.
Cheng YJ, Zhu ZX, Zhou JS, Hu ZQ, Zhang JP, Cai QP, et al. Silencing profilin-1 inhibits gastric cancer progression via integrin beta1/focal adhesion kinase pathway modulation. World J Gastroenterol. 2015;21:2323–35.
Chakraborty S, Jiang C, Gau D, Oddo M, Ding Z, Vollmer L, et al. Profilin-1 deficiency leads to SMAD3 upregulation and impaired 3D outgrowth of breast cancer cells. Br J Cancer. 2018;119:1106–17.
Zhao J, Xu J, Lv J. Identification of profilin 1 as the primary target for the anti-cancer activities of Furowanin A in colorectal cancer. Pharm Rep. 2019;71:940–9.
Jiang C, Ding Z, Joy M, Chakraborty S, Kim SH, Bottcher R, et al. A balanced level of profilin-1 promotes stemness and tumor-initiating potential of breast cancer cells. Cell Cycle. 2017;16:2366–73.
Baig MS, Roy A, Rajpoot S, Liu D, Savai R, Banerjee S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69:435–51.
Ji H, Greening DW, Kapp EA, Moritz RL, Simpson RJ. Secretome-based proteomics reveals sulindac-modulated proteins released from colon cancer cells. Proteom Clin Appl. 2009;3:433–51.
Zhao Y, Ge X, Xu X, Yu S, Wang J, Sun L. Prognostic value and clinicopathological roles of phenotypes of tumour-associated macrophages in colorectal cancer. J Cancer Res Clin Oncol. 2019;145:3005–19.
Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, et al. Regulated polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J Immunol. 2017;199:1505–15.
Acknowledgements
We would like to show sincere appreciation to the reviewers for critical comments on this article.
Author information
Authors and Affiliations
Contributions
JZ and SL designed the study. XZ, CL, and JZ collated the data, carried out data analyses, and produced the initial draft of the manuscript. JZ and WZ contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, J., Li, S., Zhang, X. et al. LncRNA HLA-F-AS1 promotes colorectal cancer metastasis by inducing PFN1 in colorectal cancer-derived extracellular vesicles and mediating macrophage polarization. Cancer Gene Ther 28, 1269–1284 (2021). https://doi.org/10.1038/s41417-020-00276-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00276-3
This article is cited by
-
Potentials as biomarker and therapeutic target of upregulated long non-coding RNA HLA-F antisense RNA 1 in hepatitis B virus-associated hepatocellular carcinoma
Virus Genes (2024)
-
The application of extracellular vesicles in colorectal cancer metastasis and drug resistance: recent advances and trends
Journal of Nanobiotechnology (2023)
-
Tumor cell-derived exosomes mediating hsa_circ_0001739/lncRNA AC159540.1 facilitate liver metastasis in colorectal cancer
Cell Biology and Toxicology (2023)
-
Constructing a Novel Signature Based on Immune-Related lncRNA to Improve Prognosis Prediction of Cervical Squamous Cell Carcinoma Patients
Reproductive Sciences (2022)
-
Oxaliplatin inhibits colorectal cancer progression by inhibiting CXCL11 secreted by cancer-associated fibroblasts and the CXCR3/PI3K/AKT pathway
Clinical and Translational Oncology (2022)