Abstract
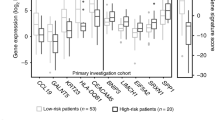
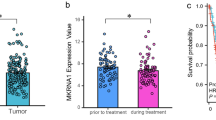
Cervical cancer is one of the most prevalent gynecologic malignancies and has remained an intractable cancer over the past decades. We analyzed the aberrant expression patterns of cervical cancer using RNA-Seq data from The Cancer Genome Atlas (TCGA). A total of 3352 differently expressed genes (DEGs) were identified in 306 cervical cancer samples compared with 3 control samples, with 1401 genes upregulated and 1951 downregulated. Under Kaplan–Meier analysis, 76 out of these DEGs with a significantly different survival rate between patients with high and low expression groups were picked out and uploaded to perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, which identified a transferrin receptor (TFRC)-involved HIF-1 signaling pathway (p < 0.05). Clinical data analysis showed that high TFRC expression in cervical cancers was associated with incrementally advanced stage, tumor status, and lymph nodes (all p-values <0.05), while multivariate analysis revealed that TFRC remained an independent prognostic variable for poor overall survival. In conclusion, our data indicated that the TFRC-involved HIF-1 signaling pathway may play a crucial role in cervical cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for Clinical Practice Guidelines. Ann Oncol. 2017;28:72–83.
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D. et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297–316.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol. 2016;27:1–12.
Sant M, Chirlaque Lopez MD, Agresti R, Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, et al. Survival of women with cancers of breast and genital organs in Europe 1999-2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51:2191–205.
Rung J, Brazma A. Reuse of public genome-wide gene expression data. Nat Rev Genet. 2013;14:1–11.
Creixell P, Reimand J, Haider S, Wu G, Shibata T, Vazquez M, et al. Pathway and network analysis of cancer genomes. Nat Methods. 2015;12:615–21.
Zhao H, Li H. Network-based meta-analysis in the identification of biomarkers for papillary thyroid cancer. Gene. 2018;661:160–8.
Yasrebi H. SurvJamda: an R package to predict patients’ survival and risk assessment using joint analysis of microarray gene expression data. Bioinformatics. 2011;27:1168–9.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Burk RD, Chen Z, Saller C, Tarvin K, Carvalho AL, Scapulatempo-Neto C, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–84.
Uyar D, Rader J. Genomics of cervical cancer and the role of human papillomavirus pathobiology. Clin Chem. 2014;60:144–6.
Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8:916–31.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastas- Rev. 2007;26:225–39.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75.
Zhang L, Huang S-T, Feng Y-L, Wan T, Gu H-F, Xu J, et al. The bidirectional regulation between MYL5 and HIF-1α promotes cervical carcinoma metastasis. Theranostics. 2017;7:3768–80.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36.
Sima N, Wang W, Kong D, Deng D, Xu Q, Zhou J, et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of Rb and p53. Apoptosis. 2008;13:273–81.
Strati K, Lambert PF. Role of Rb-dependent and Rb-independent functions of papillomavirus E7 oncogene in head and neck cancer. Cancer Res. 2007;67:11585–93.
Shenoy N, Pagliaro L. Sequential pathogenesis of metastatic VHL mutant clear cell renal cell carcinoma: putting it together with a translational perspective. Ann Oncol. 2016;27:1685–95.
Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180.
Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928–35.
Perroud B, Lee J, Valkova N, Dhirapong A, Lin PY, Fiehn O, et al. Pathway analysis of kidney cancer using proteomics and metabolic profiling. Mol Cancer. 2006;5:1–17.
Zaravinos A, Pieri M, Mourmouras N, Anastasiadou N, Zouvani I, Delakas D, et al. Altered metabolic pathways in clear cell renal cell carcinoma: A meta-analysis and validation study focused on the deregulated genes and their associated networks. Oncoscience. 2014;1:117.
Zhang Y, Ren YJ, Guo LC, Ji C, Hu J, Zhang HH, et al. Nucleus accumbens-associated protein-1 promotes glycolysis and survival of hypoxic tumor cells via the HDAC4-HIF-1α axis. Oncogene. 2017;36:4171–81.
Yong Y, Zhang C, Gu Z, Du J, Guo Z, Dong X, et al. Polyoxometalate-based radiosensitization platform for treating hypoxic tumors by attenuating radioresistance and enhancing radiation response. ACS Nano. 2017;11:7164–76.
Bachtiary B, Schindl M, Pötter R, Dreier B, Knocke TH, Hainfellner JA, et al. Overexpression of hypoxia-inducible factor 1 α indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234–40.
Brökers N, Le-Huu S, Vogel S, Hagos Y, Katschinski DM, Kleinschmidt M. Increased chemoresistance induced by inhibition of HIF-prolyl-hydroxylase domain enzymes. Cancer Sci. 2010;101:129–36.
Jiao M, Nan KJ. Activation of PI3 kinase/Akt/HIF-1?? pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int J Oncol. 2012;40:461–8.
Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed. 2014;53:3362–6.
Minegishi H, Fukashiro S, Ban HS, Nakamura H. Discovery of indenopyrazoles as a new class of hypoxia inducible factor (HIF)-1 inhibitors. ACS Med Chem Lett. 2013;4:297–301.
Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm. 2012;9:2863–74.
Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2:758–70.
Soni S, Padwad YS. HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. 2017;56:503–15.
Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. Erratum: O2 ⋅− and H2O2-mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;32:268.
Jung M, Weigert A, Mertens C, Rehwald C, Brüne B. Iron handling in tumor-associated macrophages-Is there a new role for lipocalin-2? Front Immunol. 2017;8:1–12.
Buas MF, Rho J, Chai X, Zhang Y, Lampe PD, Li CI. Candidate early detection protein biomarkers for ER + /PR + invasive ductal breast carcinoma identified using pre-clinical plasma from the WHI observational study. Breast Cancer Res Treat. 2015;153:445–54.
Song JY, Lee JK, Lee NW, Jung HH, Kim SH, Lee KW. Microarray analysis of normal cervix, carcinoma in situ, and invasive cervical cancer: identification of candidate genes in pathogenesis of invasion in cervical cancer. Int J Gynecol Cancer. 2008;18:1051–9.
Yu X, Feng L, Liu D, Zhang L, Wu B, Jiang W. Quantitative proteomics reveals the novel co-expression signatures in early brain development for prognosis of glioblastoma multiforme. Oncotarget. 2017;7:14161–71.
Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci USA. 1983;80:3494–8.
Ned RM, Swat W, Andrews NC. Transferrin receptor 1 is differentially required in lymphocyte development. Blood. 2003;102:3711–8.
Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48:74–78.
Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, et al. Immunosuppressive CD71 + erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–62.
Daniels TR, Ortiz-Sánchez E, Luria-Pérez R, Quintero R, Helguera G, Bonavida B. et al. An antibody-based multifaceted approach targeting the human transferrin receptor for the treatment of B-cell malignancies. J Immunother. 2011;34:500–8.
Daniels-Wells TR, Widney DP, Leoh LS, Martínez-Maza O, Penichet ML. Efficacy of an anti-transferrin receptor 1 antibody against AIDS-related non-Hodgkin lymphoma. J Immunother. 2015;38:307–10.
Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–58.
Gameiro SF, Zhang A, Ghasemi F, Barrett JW, Nichols AC, Mymryk JS. Analysis of class I major histocompatibility complex gene transcription in human tumors caused by human papillomavirus infection. Viruses. 2017;9:E252
Qin Y, Ekmekcioglu S, Forget M-A, Szekvolgyi L, Hwu P, Grimm EA, et al. Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators. Front Immunol. 2017;8:1–8.
Chen L, Luan S, Xia B, Liu Y, Gao Y, Yu H, et al. Integrated analysis of HPV-mediated immune alterations in cervical cancer. Gynecol Oncol. 2018;149:248–55.
Li X, Tian R, Gao H, Yang Y, Williams BRG, Gantier MP, et al. Identification of a histone family gene signature for predicting the prognosis of cervical cancer patients. Sci Rep. 2017;7:1–13.
Acknowledgements
We thank Juan Chen from CCN department of ZTE for her computer technology support. This work was supported by the Natural Science Foundation of Jiangsu Province (BK20151096) and the Key Projects of National Health and Family Planning Commission of Nanjing City (ZKX17015) to H.Z. This work was also supported by the Jiangsu Provincial Science and Technology Department (No. BE2012606) to Y.H.
Author contributions
X.X., Y.H., and H.Z. designed the study, checked the data, and prepared the manuscript; T.L. and Y.W. performed data collection and statistical analysis. J.W. searched the literature and took part in the manuscript preparation. Y.H. and H.Z. conceived and supervised the project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, X., Liu, T., Wu, J. et al. Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer. Cancer Gene Ther 26, 356–365 (2019). https://doi.org/10.1038/s41417-019-0078-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0078-x
This article is cited by
-
New mechanisms and biomarkers of lymph node metastasis in cervical cancer: reflections from plasma proteomics
Clinical Proteomics (2023)
-
Ferritinophagy: research advance and clinical significance in cancers
Cell Death Discovery (2023)
-
Landscape and Construction of a Novel N6-methyladenosine-related LncRNAs in Cervical Cancer
Reproductive Sciences (2023)
-
Differential proteomics reveals overexpression of ferroptosis-related proteins in cervical cancer tissue
Journal of Proteins and Proteomics (2023)
-
Iron metabolism protein transferrin receptor 1 involves in cervical cancer progression by affecting gene expression and alternative splicing in HeLa cells
Genes & Genomics (2022)