Abstract
Background
CC-115, a dual mTORC1/2 and DNA-PK inhibitor, has promising antitumour activity when combined with androgen receptor (AR) inhibition in pre-clinical models.
Methods
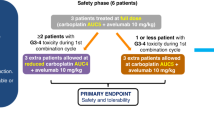
Phase 1b multicentre trial evaluating enzalutamide with escalating doses of CC-115 in AR inhibitor-naive mCRPC patients (n = 41). Primary endpoints were safety and RP2D. Secondary endpoints included PSA response, time-to-PSA progression, and radiographic progression.
Results
Common adverse effects included rash (31.7% Grades 1–2 (Gr); 31.7% Gr 3), pruritis (43.9% Gr 1–2), diarrhoea (37% Gr 1–2), and hypertension (17% Gr 1–2; 9.8% Gr 3). CC-115 RP2D was 5 mg twice a day. In 40 evaluable patients, 80% achieved ≥50% reduction in PSA (PSA50), and 58% achieved ≥90% reduction in PSA (PSA90) by 12 weeks. Median time-to-PSA progression was 14.7 months and median rPFS was 22.1 months. Stratification by PI3K alterations demonstrated a non-statistically significant trend towards improved PSA50 response (PSA50 of 94% vs. 67%, p = 0.08). Exploratory pre-clinical analysis suggested CC-115 inhibited mTOR pathway strongly, but may be insufficient to inhibit DNA-PK at RP2D.
Conclusions
The combination of enzalutamide and CC-115 was well tolerated. A non-statistically significant trend towards improved PSA response was observed in patients harbouring PI3K pathway alterations, suggesting potential predictive biomarkers of response to a PI3K/AKT/mTOR pathway inhibitor.
Clinical trial registration
ClinicalTrials.gov identifier: NCT02833883.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
American Cancer Society. Cancer Statistics Center. 2022. http://cancerstatisticscenter.cancer.org.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. https://doi.org/10.1056/NEJMoa1405095.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. https://doi.org/10.1056/NEJMoa1014618.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11. https://doi.org/10.1038/nrc4016.
Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805. https://doi.org/10.1158/1078-0432.CCR-08-0125.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. https://doi.org/10.1016/j.ccr.2011.04.008.
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. https://doi.org/10.1016/j.ccr.2011.05.006.
Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15:222–34. https://doi.org/10.1038/nrurol.2018.9.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. https://doi.org/10.1016/j.cell.2015.05.001.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. https://doi.org/10.1016/j.ccr.2010.05.026.
Rescigno P, Lorente D, Dolling D, Ferraldeschi R, Rodrigues DN, Riisnaes R, et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. Eur Urol Oncol. 2018;1:71–7. https://doi.org/10.1016/j.euo.2018.02.006.
Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015;67:795–802. https://doi.org/10.1016/j.eururo.2014.10.027.
Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398:131–42. https://doi.org/10.1016/S0140-6736(21)00580-8.
Mohiuddin IS, Kang MH. DNA-PK as an emerging therapeutic target in cancer. Front Oncol. 2019;9:635 https://doi.org/10.3389/fonc.2019.00635.
Yue X, Bai C, Xie D, Ma T, Zhou PK. DNA-PKcs: a multi-faceted player in DNA damage response. Front Genet. 2020;11:607428 https://doi.org/10.3389/fgene.2020.607428.
Dylgjeri E, McNair C, Goodwin JF, Raymon HK, McCue PA, Shafi AA, et al. Pleiotropic impact of DNA-PK in cancer and implications for therapeutic strategies. Clin Cancer Res. 2019;25:5623–37. https://doi.org/10.1158/1078-0432.CCR-18-2207.
Goodwin JF, Kothari V, Drake JM, Zhao S, Dylgjeri E, Dean JL, et al. DNA-PKcs-mediated transcriptional regulation drives prostate cancer progression and metastasis. Cancer Cell. 2015;28:97–113. https://doi.org/10.1016/j.ccell.2015.06.004.
Bouchaert P, Guerif S, Debiais C, Irani J, Fromont G. DNA-PKcs expression predicts response to radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1179–85. https://doi.org/10.1016/j.ijrobp.2012.02.014.
Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71. https://doi.org/10.1158/2159-8290.CD-13-0108.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53. https://doi.org/10.1158/2159-8290.CD-13-0172.
Tsuji T, Sapinoso LM, Tran T, Gaffney B, Wong L, Sankar S, et al. CC-115, a dual inhibitor of mTOR kinase and DNA-PK, blocks DNA damage repair pathways and selectively inhibits ATM-deficient cell growth in vitro. Oncotarget. 2017;8:74688–702. https://doi.org/10.18632/oncotarget.20342.
Munster P, Mita M, Mahipal A, Nemunaitis J, Massard C, Mikkelsen T, et al. First-in-human phase I study of a dual mTOR kinase and DNA-PK inhibitor (CC-115) in advanced malignancy. Cancer Manag Res. 2019;11:10463–76. https://doi.org/10.2147/CMAR.S208720.
Sweeney CJ, Percent IJ, Babu S, Cultrera JL, Mehlhaff BA, Goodman OB, et al. Phase 1b/2 study of enzalutamide with samotolisib (LY3023414) or placebo in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2022 https://doi.org/10.1158/1078-0432.CCR-21-2326.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Schonhoft JD, Zhao JL, Jendrisak A, Carbone EA, Barnett ES, Hullings MA, et al. Morphology-predicted large-scale transition number in circulating tumor cells identifies a chromosomal instability biomarker associated with poor outcome in castration-resistant prostate cancer. Cancer Res. 2020;80:4892–903. https://doi.org/10.1158/0008-5472.CAN-20-1216.
Brown LC, Halabi S, Schonhoft JD, Yang Q, Luo J, Nanus DM, et al. Circulating tumor cell chromosomal instability and neuroendocrine phenotype by immunomorphology and poor outcomes in men with mCRPC treated with abiraterone or enzalutamide. Clin Cancer Res. 2021;27:4077–88. https://doi.org/10.1158/1078-0432.CCR-20-3471.
Scher HI, Armstrong AJ, Schonhoft JD, Gill A, Zhao JL, Barnett E, et al. Development and validation of circulating tumour cell enumeration (Epic Sciences) as a prognostic biomarker in men with metastatic castration-resistant prostate cancer. Eur J Cancer. 2021;150:83–94. https://doi.org/10.1016/j.ejca.2021.02.042.
Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1441–9. https://doi.org/10.1001/jamaoncol.2016.1828.
Greene SB, Dago AE, Leitz LJ, Wang Y, Lee J, Werner SL, et al. Chromosomal instability estimation based on next generation sequencing and single cell genome wide copy number variation analysis. PLoS ONE. 2016;11:e0165089 https://doi.org/10.1371/journal.pone.0165089.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13. https://doi.org/10.1038/nm.4333.
Annala M, Taavitsainen S, Khalaf DJ, Vandekerkhove G, Beja K, Sipola J, et al. Evolution of castration-resistant prostate cancer in ctDNA during sequential androgen receptor pathway inhibition. Clin Cancer Res. 2021;27:4610–23. https://doi.org/10.1158/1078-0432.CCR-21-1625.
Scher HI, Graf RP, Schreiber NA, McLaughlin B, Jendrisak A, Wang Y, et al. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res. 2017;77:5687–98. https://doi.org/10.1158/0008-5472.CAN-17-1353.
Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djx118.
Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–57. https://doi.org/10.1158/2159-8290.CD-17-0937.
Pan A, Reingold RE, Zhao JL, Moy A, Kraehenbuehl L, Dranitsaris G, et al. Dermatological adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010–9. https://doi.org/10.1097/JU.0000000000002425.
Deng M, Chai H, Yang M, Wei X, Zhang W, Wang X, et al. Stevens-Johnson syndrome caused by enzalutamide: a case report and literature review. Front Oncol. 2021;11:736975 https://doi.org/10.3389/fonc.2021.736975.
FDA. Xtandi (enzalutamide) prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203415lbl.pdf.
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2013;8:e55128 https://doi.org/10.1371/journal.pone.0055128.
Schweizer MT, Ha G, Gulati R, Brown LC, McKay RR, Dorff T, et al. CDK12-mutated prostate cancer: clinical outcomes with standard therapies and immune checkpoint blockade. JCO Precis Oncol. 2020;4:382–92. https://doi.org/10.1200/po.19.00383.
Antonarakis ES, Isaacsson Velho P, Fu W, Wang H, Agarwal N, Sacristan, et al. CDK12-altered prostate cancer: clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-ribose) polymerase inhibitors, and PD-1 inhibitors. JCO Precis Oncol. 2020;4:370–81. https://doi.org/10.1200/po.19.00399.
Hamid AA, Gray KP, Shaw G, MacConaill LE, Evan C, Bernard B, et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol. 2019;76:89–97. https://doi.org/10.1016/j.eururo.2018.11.045.
Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7:736–49. https://doi.org/10.1158/2159-8290.CD-16-1174.
Wang Y, Wang Y, Ci X, Choi SYC, Crea F, Lin D, et al. Molecular events in neuroendocrine prostate cancer development. Nat Rev Urol. 2021;18:581–96. https://doi.org/10.1038/s41585-021-00490-0.
Acknowledgements
We thank all the patients, their families, and the study site investigators and staff who participated in the study.
Funding
The study was supported and funded by Celgene/BMS and Gateway for Cancer Research. The trial was managed by Prostate Cancer Clinical Trials Consortium (PCCTC). JLZ was supported by a K12 Paul Calabresi Career Development Award for Clinical Oncology and a Prostate Cancer Foundation Young Investigator Award.
Author information
Authors and Affiliations
Contributions
JLZ and ESA designed the study, acquired the data, interpreted the results, and drafted and revised the manuscript. HHC, DJG, RA, WA, FYF, KK, MEL and AWW designed the study, acquired the data, interpreted the results, and revised the manuscript. ER designed statistical methods, interpreted the results and drafted and revised the manuscript. TS designed the ctDNA analysis, acquired the data, interpreted the results and revised the manuscript. JDS and AA designed the CTC analysis, acquired the data, interpreted the results and revised the manuscript. NM, SH and BC designed the pre-clinical experiment, acquired the data, interpreted the results and revised the manuscript. BD and TC acquired the data, interpreted the results, and revised the manuscript. DR corresponding author, supervised the study, designed the study, acquired the data, interpreted the results, and drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
JLZ is currently a full-time employee at AstraZeneca, which is not involved in the funding and conduct of the trial. The authors declare no competing interests.
Ethics approval and consent to participate
IRB committee approval was obtained from all participating sites and written consent was obtained by all participating patients. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41416_2023_2487_MOESM2_ESM.pdf
Table S1. Summary table of PI3K, TP53 and DNA repair pathway mutations by next-generation sequencing (NGS) and PSA response by 12 weeks
41416_2023_2487_MOESM6_ESM.pdf
Figure S4. Pre-clinical biochemical analysis of target pathway inhibition (A) and in vitro cell proliferation (B and C).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J.L., Antonarakis, E.S., Cheng, H.H. et al. Phase 1b study of enzalutamide plus CC-115, a dual mTORC1/2 and DNA-PK inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC). Br J Cancer 130, 53–62 (2024). https://doi.org/10.1038/s41416-023-02487-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02487-5