Abstract
Background
Dickkopf-related protein 1 (DKK1) is a Wingless-related integrate site (Wnt) signaling modulator that is upregulated in prostate cancers (PCa) with low androgen receptor expression. DKN-01, an IgG4 that neutralizes DKK1, delays PCa growth in pre-clinical DKK1-expressing models. These data provided the rationale for a clinical trial testing DKN-01 in patients with metastatic castration-resistant PCa (mCRPC).
Methods
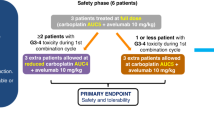
This was an investigator-initiated parallel-arm phase 1/2 clinical trial testing DKN-01 alone (monotherapy) or in combination with docetaxel 75 mg/m2 (combination) for men with mCRPC who progressed on ≥1 AR signaling inhibitors. DKK1 status was determined by RNA in-situ expression. The primary endpoint of the phase 1 dose escalation cohorts was the determination of the recommended phase 2 dose (RP2D). The primary endpoint of the phase 2 expansion cohorts was objective response rate by iRECIST criteria in patients treated with the combination.
Results
18 pts were enrolled into the study—10 patients in the monotherapy cohorts and 8 patients in the combination cohorts. No DLTs were observed and DKN-01 600 mg was determined as the RP2D. A best overall response of stable disease occurred in two out of seven (29%) evaluable patients in the monotherapy cohort. In the combination cohort, five out of seven (71%) evaluable patients had a partial response (PR). A median rPFS of 5.7 months was observed in the combination cohort. In the combination cohort, the median tumoral DKK1 expression H-score was 0.75 and the rPFS observed was similar between patients with DKK1 H-score ≥1 versus H-score = 0.
Conclusion
DKN-01 600 mg was well tolerated. DKK1 blockade has modest anti-tumor activity as a monotherapy for mCRPC. Anti-tumor activity was observed in the combination cohorts, but the response duration was limited. DKK1 expression in the majority of mCRPC is low and did not clearly correlate with anti-tumor activity of DKN-01 plus docetaxel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data on which the conclusions of the paper rely are presented in the main manuscript and the additional supporting material.
References
Sartor O, de Bono JS. Metastatic prostate cancer. N. Engl J Med. 2018;378:645–57.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl J Med. 2004;351:1502–12.
Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. NCCN Guidelines® insights: prostate cancer, Version 1.2023. J Natl Compr Cancer Netw. 2022;20:1288–98.
Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943–7.
Crabb SJ, Griffiths G, Marwood E, Dunkley D, Downs N, Martin K, et al. Pan-AKT inhibitor capivasertib with docetaxel and prednisolone in metastatic castration-resistant prostate cancer: a randomized, placebo-controlled phase II Trial (ProCAID). J Clin Oncol. 2021;39:190–201.
Petrylak DP, Ratta R, Matsubara N, Korbenfeld EP, Gafanov R, Mourey L, et al. Pembrolizumab plus docetaxel for patients with metastatic castration-resistant prostate cancer (mCRPC): randomized, double-blind, phase 3 KEYNOTE-921 study. J Clin Oncol. 2023;41:19.
Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl J Med. 2014;371:1028–38.
Robinson D, Van Allen EliezerM, Wu Y-M, Schultz N, Lonigro RobertJ, Mosquera J-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Abida W CJ, Heller G, Prandic D, Armenia J, Coleman I, Cieslik M, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci. 2019;116:11428–36.
Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–89.e6.
Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95.
Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50.
Aparicio A, Logothetis CJ, Maity SN. Understanding the lethal variant of prostate cancer: power of examining extremes. Cancer Discov. 2011;1:466–8.
Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–30.
Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–30.
Nyquist MD, Corella A, Coleman I, De Sarkar N, Kaipainen A, Ha G, et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 2020;31:107669.
Wise DR, Schneider JA, Armenia J, Febles VA, McLaughlin B, Brennan R, et al. Dickkopf-1 can lead to immune evasion in metastatic castration-resistant prostate cancer. JCO Precis Oncol. 2020;4:1167–79.
Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–25.
Kagey MH, He X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br J Pharm. 2017;174:4637–50.
Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H, et al. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Investig. 2016;126:2689–705.
Hall CL, Zhang H, Baile S, Ljungman M, Kuhstoss S, Keller ET. p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1. Cancer Res. 2010;70:9916–26.
Aggarwal R, Romero GR, Friedl V, Weinstein A, Foye A, Huang J, et al. Clinical and genomic characterization of Low PSA Secretors: a unique subset of metastatic castration resistant prostate cancer. Prostate Cancer Prostat Dis. 2021;24:81–7.
Bendell JC, Murphy JE, Mahalingam D, Halmos B, Sirard CA, Landau SB, et al. Phase I study of DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (pac) in patients (pts) with DKK1+ relapsed or refractory esophageal cancer (EC) or gastro-esophageal junction tumors (GEJ). J Clin Oncol. 2016;34:111.
Klempner SJ, Bendell JC, Villaflor VM, Tenner LL, Stein SM, Rottman JB, et al. Safety, efficacy, and biomarker results from a phase Ib study of the Anti-DKK1 antibody DKN-01 in combination with pembrolizumab in advanced esophagogastric cancers. Mol Cancer Ther. 2021;20:2240–9.
Simon R. Optimal two-stage designs for phase II clinical trials. Controll Clin Trials. 1989;10:1–10.
Oudard S, Fizazi K, Sengeløv L, Daugaard G, Saad F, Hansen S, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III Trial-FIRSTANA. J Clin Oncol. 2017;35:3189–97.
Sachdev A, Sharpe I, Bowman M, Booth CM, Gyawali B. Objective response rate of placebo in randomized controlled trials of anticancer medicines. EClinicalMedicine. 2023;55:101753.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e52.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working Group 3. J Clin Oncol. 2016;34:1402–18.
Yang GC, Wan LS, Papellas J, Waisman J. Compact cell blocks. Use for body fluids, fine needle aspirations and endometrial brush biopsies. Acta Cytol. 1998;42:703–6.
Betella I, Turbitt WJ, Szul T, Wu B, Martinez A, Katre A, et al. Wnt signaling modulator DKK1 as an immunotherapeutic target in ovarian cancer. Gynecol Oncol. 2020;157:765–74.
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–6.
Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67.
Hu Z, Zhou J, Li Y, Luan Y, Li H, Jia B, et al. Peripheral immune signature resembles tumor microenvironment and predicts clinical outcomes in head and neck squamous cell carcinoma. Front Immunol. 2022;13:915207.
Rad Pour S, Pico de Coaña Y, Martinez de Morentin Iribarren X, Melief J, Thimma M, Wolodarski M, et al. Predicting anti-PD-1 responders in malignant melanoma from the frequency of S100A9+ monocytes in the blood. J Immunother Cancer. 2021;9:e002171.
Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, et al. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology. 2012;136:176–83.
Song R, Liu F, Ping Y, Zhang Y, Wang L. Potential non-invasive biomarkers in tumor immune checkpoint inhibitor therapy: response and prognosis prediction. Biomark Res. 2023;11:57.
Krause U, Ryan DM, Clough BH, Gregory CA. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis. 2014;5:e1093.
Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, et al. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71:615–25.
Smadja DM, d’Audigier C, Weiswald LB, Badoual C, Dangles-Marie V, Mauge L, et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol. 2010;30:2544–52.
Malladi S, Macalinao DaniloG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60.
D’Amico L, Mahajan S, Capietto AH, Yang Z, Zamani A, Ricci B, et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med. 2016;213:827–40.
Klempner SJ, Chao J, Uronis HE, Sirard CA, Kagey M, Baum J, et al. DKN-01 and tislelizumab ± chemotherapy as a first-line (1L) and second-line (2L) investigational therapy in advanced gastroesophageal adenocarcinoma (GEA): DisTinGuish Trial. J Clin Oncol. 2022;40:292.
Arend RC, Castro CM, Matulonis UA, Hamilton E, Gunderson CC, Lybarger KSS, et al. Dkn-01 treated patients with recurrent epithelial endometrial (EEC) or ovarian (EOC) cancers which harbor Wnt activating mutations have longer progression-free survival and improved clinical benefit. Gynecol Oncol. 2020;159:5–6.
Chen L, Zhou Q, Liu J, Zhang W. CTNNB1 alternation is a potential biomarker for immunotherapy prognosis in patients with hepatocellular carcinoma. Front Immunol. 2021;12:759565.
Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–3.
Akasu M, Shimada S, Kabashima A, Akiyama Y, Shimokawa M, Akahoshi K, et al. Intrinsic activation of β-catenin signaling by CRISPR/Cas9-mediated exon skipping contributes to immune evasion in hepatocellular carcinoma. Sci Rep. 2021;11:16732.
Nsengimana J, Laye J, Filia A, O’Shea S, Muralidhar S, Poźniak J, et al. β-Catenin–mediated immune evasion pathway frequently operates in primary cutaneous melanomas. J Clin Investig. 2018;128:2048–63.
Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019;25:3074–83.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231.
Shi T, Zhang Y, Wang Y, Song X, Wang H, Zhou X, et al. DKK1 promotes tumor immune evasion and impedes Anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. 2022;10:1506–24.
Park W-J, Kim MJ. A new wave of targeting ‘undruggable’ wnt signaling for cancer therapy: challenges and opportunities. Cells 2023;12:1110.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12.
Chou J, Egusa EA, Wang S, Badura ML, Lee F, Bidkar AP, et al. Immunotherapeutic targeting and PET imaging of DLL3 in small-cell neuroendocrine prostate cancer. Cancer Res. 2023;83:301–15.
Plas S, Pircher A, Heidegger I. Pembrolizumab in mCRPC – combination therapies as breakthrough to success? Curr Opin Urol. 2023;33:458–71.
Arend R, Dholakia J, Castro C, Matulonis U, Hamilton E, Jackson CG, et al. DKK1 is a predictive biomarker for response to DKN-01: results of a phase 2 basket study in women with recurrent endometrial carcinoma. Gynecol Oncol. 2023;172:82–91.
Parsons MJ, Tammela T, Dow LE. WNT as a driver and dependency in cancer. Cancer Discov. 2021;11:2413–29.
Acknowledgements
We acknowledge the patients, their families, and caregivers for their participation in this study. We acknowledge the NYULH Center for Biospecimen Research and Development (RRID: SCR_017930) and Histology and Immunohistochemistry Laboratory (RRID:SCR_018304) supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087. This work was supported in part by a Prostate Cancer Foundation Young Investigator Award E2457556 to DRW.
Author information
Authors and Affiliations
Contributions
Conceptualization: DRW, JD, AVB, MHK, WN, ABT, PC, CA, KKW. Collection and assembly of data: DRW, RKP, SRD, RRA, JD, VAF, CL, SS, SG, DL, NY, VA, SM, EW, GG, NS, AP, EF, FD, QR, LC, JM. Data analysis and interpretation: DRW, RKP, JD, AVB, MH, MHK, WN, JB, ABT, AP, PC, FMD, JM, CA. Paper writing-original draft: DRW, JB, CA. Paper review and editing: All authors.
Corresponding author
Ethics declarations
Competing interests
DRW is a paid consultant for Leap Therapeutics, Foundation Medicine, Pfizer, Janssen, Sanofi, Lilly, Labcorp, Myovant, Bayer, AstraZeneca, Accutar. He has received travel funding from Pfizer and Bayer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wise, D.R., Pachynski, R.K., Denmeade, S.R. et al. A Phase 1/2 multicenter trial of DKN-01 as monotherapy or in combination with docetaxel for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00798-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00798-z