Abstract
Background
Molecular Tumour Boards (MTBs) were created with the purpose of supporting clinical decision-making within precision medicine. Though in use globally, reporting on these meetings often focuses on the small percentages of patients that receive treatment via this process and are less likely to report on, and assess, patients who do not receive treatment.
Methods
A literature review was performed to understand patient attrition within MTBs and barriers to patients receiving treatment. A total of 51 papers were reviewed spanning a 6-year period from 11 different countries.
Results
In total, 20% of patients received treatment through the MTB process. Of those that did not receive treatment, the main reasons were no mutations identified (27%), no actionable mutations (22%) and clinical deterioration (15%). However, data were often incomplete due to inconsistent reporting of MTBs with only 55% reporting on patients having no mutations, 55% reporting on the presence of actionable mutations with no treatment options and 59% reporting on clinical deterioration.
Discussion
As patient attrition in MTBs is an issue which is very rarely alluded to in reporting, more transparent reporting is needed to understand barriers to treatment and integration of new technologies is required to process increasing omic and treatment data.
Similar content being viewed by others
Introduction
The human genome project provided the world with a fully referenced genome that helped to illuminate the role of somatic and germline mutations in the pathogenesis of cancer [1]. The development of next-generation sequencing (NGS) propelled genomics research even further, enabling the sequencing of entire genomes within days rather than decades. This helped facilitate the use of genomics sequencing within clinically meaningful timelines and identify aberrant pathways for the development of new and effective targeted treatment options for patients [2], facilitating rapid precision medicine on a larger scale [3]. Precision medicine is a healthcare model that allows treatment to be tailored to individuals by categorising them into genomic subpopulations [4]. Precision medicine is reliant on the knowledge and expertise of coordinating specialities, requiring persistent adoption of rapidly advancing science and new techniques.
Targeted therapies are drugs that target specific genes or proteins in cancerous cells [5]. Lung cancer treatment has had a number of successes with targeted therapy; drugs targeting EGFR mutations and ALK and ROS1 translocations are now routinely used in cancer treatment [6]. However, precision medicine successes are not straightforward, for example, before Crizotinib was licenced for use in ALK translocations, it was originally tested in MET mutated tumours [7]. Despite having potent activity against MET [8], studies found no anti-tumour effect in tumours with MET mutations, though, subsequently demonstrated efficacy against tumours with MET exon 14 skipping alterations [9]. Often determining appropriate targeted therapies for patients with pathogenic mutations requires input from multiple disciplines, therefore, organisations regularly consult with or develop Molecular Tumour Boards (MTB). Molecular Tumour Boards, Precision Genomics Boards or Genomics Review Boards are all names for a multidisciplinary team that consult on individual patients’ treatment options, either providing expert opinion to healthcare professionals who have limited access to multidisciplinary expertise or driving decisions for their own patients. These meetings focus on patients with rare, hard-to-treat or late-stage malignant disease and are composed of various specialists but always include oncologists or clinicians and scientists or biologists (see Supplementary references). Clinical research has shown that these teams can help facilitate precision medicine, however, with increased evaluation limitations have emerged [10, 11].
MTBs were developed for the specific purpose of supporting complex clinical decision-making and are often only reported in terms of positive outcomes. However, it is imperative that we are cognisant of the outcomes of patients who never reach the treatment phase on these pathways, to ensure that we are striving to improve processes and therefore opportunities for patients.
The objective of this review is to assess how global MTBs are conducted and identify common reasons for the lack of treatment options, evaluating whether there are procedural issues that contribute to this attrition and areas for potential process optimisation. In addition, suggested guidelines for the future reporting of MTBs may be informed by this review.
These guidelines could allow for transparent and consistent reporting, bringing awareness to deficiencies in the current system and facilitating change to mitigate against attrition and to ensure that all patients are given the greatest opportunity to access treatments.
The contribution of this paper is:
-
Quantification of the issues with MTBs.
-
A description of the reasons for patient attrition in an MTB.
-
Recommendations for guidelines for optimal reporting of MTBs.
Methodology
Literature-based analysis
A review of published literature was performed to evaluate current MTB processes and understand the reasons a treatment option is not identified or accessed by a patient after review in an MTB. Databases searched were EMBASE and PubMed, and were last accessed on November 19, 2020. Search terms were formalised for reproducibility purposes (see Table 1). Data collection was completed by a single author.
Inclusion criteria were:
-
written in English
-
more than five patients were reviewed through the MTB
-
multi-gene panel
-
humans only
-
and MTBs were either self-identified by the authors of the paper or were defined as a multidisciplinary team meeting that performed and reviewed multi-omic testing outside of the standard of care, on patients with cancer, with an aim to finding a targeted therapy.
Exclusion criteria were:
-
studies earlier than 2014
-
non-oncology studies
-
case studies
-
imaging studies
-
biomarker reviews
-
evaluated specific treatment regimens or focused on specific mutations only
-
an abstract, unless it supported a full paper
-
had no centralised review of patients e.g., MTB
-
no intention to treat
-
or reported only in percentages making total numbers impossible to determine.
The country the MTB was based was recorded as well as the type of institution the MTB was held at, eligible cancer types, duration of the MTB, method of genomic testing performed, variant allele fraction threshold for action, and reasoning why patients were unable to access treatments.
Where numbers were unclear or reasons for attrition were grouped, these were excluded from the analysis.
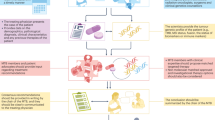
Process flow
Using the papers selected for review, a systematic formalisation of the MTB process was created using papers which described their MTB patient pathway. At the end of the review, all process flows were assimilated to create a universal structure. The flows included the patient journey from consent to tissue acquisition and analysis, the point at which patients were discussed at an MTB, the return of their full genomic results and how the results were disseminated.
The following categories for attrition were identified from the review: insufficient tissue; no mutations identified; no actionable mutations identified; actionable mutations identified but no treatment available; actionable mutations identified but ineligible for treatment; the patient had already received the matched drug; off-licence treatment available but could not access; clinically deteriorated and patients were categorised accordingly. As each study did not report on all these categories there were multiple missing data points.
Statistical analysis
Descriptive statistics only are provided due to the number of missing data points a more formal statistical review was deemed inappropriate. Patient attrition is described using percentages, as all papers did not report on each reason for attrition the percentages were derived only from papers that reported on them.
Results
A literature review gave a fuller understanding of the global picture for patients and provided insight on the perceived importance by researchers of patient attrition. The review produced over 8000 targeted results (EMBASE 115/ PubMed 7888) which was reduced to 51 evaluable papers using the exclusion and inclusion criteria listed in the methods (see Fig. 1). All reviews and data collection were performed by a single reviewer.
Study characteristics
A summary of MTB characteristics can be found in Table 2. Exactly half of all studies enrolled 100 patients or fewer to an MTB, with an overall range of 14–3737. The average study length was 30 months (range 9–60 months).
Process flow
The overarching process flow was used to identify steps in the process where there is typically patient attrition (Fig. 2). Few MTBs had unique processes, those that did differ varied by bioinformatic pipeline and whether patients were presented to the MTB before and after profiling or after only. Other areas where MTBs differed were in how the results were disseminated. This was typically done in one or more of the following routes; through an online database; via patient health records; through email or phone call to the patient; or within a report given to the treating clinician. In four of the 51 studies, patients underwent genomic testing prior to entry into an MTB and MTBs were utilised by referring clinicians for expert opinion.
As not all studies reported on all the reasons outlined in this review, percentages were calculated out of the studies where the data was available. The patient flow was created by incorporating the individual flows from the literature; not all papers provided these. The only part of the flow that changed between MTBs were the need for a review prior to the MTB to determine suitability for genomic testing, the frequency of the MTB and how results were disseminated. MTB Molecular Tumour Board.
Patient attrition
A total of 19,430 cases were described within the selected papers. Of these, the majority were adults (n = 16,171), with 628 childhood cancer cases reported. In total, 2631 cases were reported in a mixed adult and paediatric MTB. The reasons for not receiving therapy were inconsistently reported in the published literature and patient numbers reduced without explanation, in total 5673 patients had an unknown outcome (29%) (see Fig. 3), therefore there is variability in data available for patient outcomes.
Of those cases where the outcome was known, the most common reasons reported for patient attrition were: no mutations detected (27%), no actionable mutations detected (22%), clinical deterioration (15%) or lack of tissue (14%). The reason for the greatest number of patients not receiving treatment in paediatric trials (14%) was no actionable mutations, whereas in adult trials, there were no mutations and no actionable mutations.
In MTBs describing adult patients, where the data was available, 23% (3229/14338) went on to treatment; in paediatric studies, 13% (81/628) of patients went on to treatment, and in MTBs where patient populations were both adults and paediatrics 7% (173/2631) went on to treatment. The greatest rates of attrition were due to clinical deterioration, no mutations detected, or no actionable mutations detected.
Reporting of MTBs
MTBs were inconsistently reported in the literature (see Table 3). Although arguably reasons for patient attrition may vary, some studies failed to report on the number of patients treated based on recommendations by MTBs (n = 5). Also missing was the composition of the MTB (n = 12) and tissue type used for sequencing (n = 12). Only 12 MTBs reported on the presence or absence of a cut-off for variant allele frequency (VAF) and 15 reported on what actionability scales were used. Outlined in Fig. 2 are the areas in the process where patient attrition occurs and how often this was reported in the literature. Of the data that was available overall 49% of patients had an actionable mutation after genomic profiling and 20% of all patients registered to MTB received recommended treatment. There were no studies that reported on all areas defined in this review.
Discussion
Molecular tumour boards were developed to assist with assessment of genomic tests to facilitate targeted treatment for patients and have been widely implemented throughout the globe. On average, 20% of patients enrolled on an MTB received MTB-directed therapy. When able to access treatment, overall response rates vary from 0 to 67% [12]. MTBs facilitate enrolment of patients on to treatments or trials with biological potential or for their specific tumour type, some may argue that it gives patients the chance, however small, to receive life-extending drugs. However, with attrition rates exceedingly high and response rates variable, one patient in 5 will have the opportunity to access a targeted therapy after exhausting the standard of care. In addition, MTBs also provide educational opportunities for healthcare professionals, therefore, despite low patient numbers accessing treatment, there is the benefit of training professionals for the future.
As we elicited, there are two key areas of issue with treating patients through an MTB, there are high rates of patient attrition, and secondly there are low response rates. Clinical outcomes are out of the scope of this review, though are covered in detail by Larson et al. [12]. Patient attrition can be summarised into broad categories such as, lack of suitable tissue; no mutations; or actionable mutations but unable to access treatment as not available or; unable to access treatment even though available, and clinical deterioration. Practically addressing specific blockers could facilitate more patients gaining access to treatment, which we address in the following sections.
Points of intervention
Lack of tissue
All studies in this review, where the sample type used for profiling was specified, used either archived tissue samples, fresh biopsy, or surgical samples as the source of tumour profiling. Where blood samples were taken, this was usually only for germline analysis. Insufficient tissue was one of the most common reasons provided for patient attrition and comprised 14% of all patients (n = 1991) in this review. This did not include number of patients that were ineligible based upon lack of tissue as they were never enrolled in the MTB. However, to put this into context and highlight the proportion of patients this may omit, in one study alone 3290 patients failed screening due to a lack of tissue and just 229 were enrolled [13].
Liquid biopsies have been a long-awaited tool in oncology. They have been shown to be clinically relevant for different cancer types though there is still much work left to do until they can be utilised routinely by oncologists [14,15,16,17]. However, there is a clear need for alternatives to tissue analysis. Of the four studies that looked at using liquid biopsy for genomic analysis, turnaround time data are not available but only 4% (range 0–8%) of patients were ineligible due to a lack of or failure of a sample [18, 19] compared to 14% (range 0–23%) where analysis failed when only tissue was used (see Supplementary Material Table 1). Circulating free tumour-derived DNA can be used for the assessment of cancer-specific somatic mutations, chromosomal abnormalities, copy number alterations and epigenetic modifications and is elevated in malignancy [20]. It has been shown to be useful in therapy selection for patients, particularly in settings where patients are late stage [21, 22]. Furthermore, the downsides to tumour biopsies are well documented [23] and retrieving archived samples can often cause significant delays. Implementing more wide-scale liquid biopsy testing could improve the rates of attrition where patients are lacking sufficient tissue, are unsuitable for biopsy or have old archival samples.
No actionable mutations or no mutations
The two greatest reasons for patient attrition within this review were that patients’ sample yielded no mutations or no actionable mutations as determined by the reviewing MTB. It has been shown that more comprehensive multi-omics profiling provides more clinically relevant information [24,25,26], however, it is necessary to couple this with technologies that will help to prioritise the inevitable volumes of information produced [27].
The number of patients accessing treatment changes depending on the types and number of profiling tests that are performed on their sample (e.g. NGS, IHC RNAseq etc.). Interestingly there is an almost 50% decrease in treatment access when two tests are used, and treatment rates do not increase significantly the more complex testing is used (see Supplementary Material Table 2). This could be potentially explained by a lack of drugs associated with these genomic alterations or possibly due to the increased challenge for clinicians at being able to discern potentially actionable mutations due to an excess of the data. Developing computational methods to help integrate and interpret data from multiple tests to link with current literature and available treatment options could help to manage the demand on clinicians. A lack of drug availability is also a limiting factor that has the potential to get better over time [28].
Actionable mutations but ineligible or no options for treatment
Attrition due to ineligibility for clinical trials or no further treatment options available, both within a clinical trial or using off-licence treatment, affected 15% of all patients in this review. However, this was only reported in 13% (off-licence treatment available but unable to access) and 31–32% (actionable but no treatment/ trial available or ineligible for treatment) of studies so has the potential to impact greater numbers. Unsurprisingly, a common barrier to paediatric studies was gaining access to treatment options. Though drug development has increased in this area [29] further work is being done in cancers where presentation in adults differs to children or paediatric-specific cancers [30].
Eligibility criteria is important to ensure the safety of participants on clinical studies; however, it can be restrictive, resulting in the unjustified exclusion of patients from enrolment into clinical trials [31,32,33,34]. A systematic review of randomised controlled trials found that 47.2% of the criteria were not scientifically justified [33]. Not only does this result in failed recruitment for studies but also fails to evaluate efficacy and safety in real-world populations, and importantly excludes patients from receiving potential treatment options. Therefore, more flexible data-driven eligibility criteria are required to prevent unnecessary exclusion of patients from trials [35, 36], though this will require a wider consensus to drive this change. Importantly, artificial intelligence and machine learning can play a crucial role in evaluating suitable patients for studies that do not follow a restrictive exclusion/inclusion approach [34].
Identifying suitable treatment or trial options for patients can be a difficult and onerous task given a large number of recruiting studies, potentially actionable mutations and literature-based evidence currently available, which is steadily growing [37]. Therefore, trial matching software can help clinicians review available studies based upon patients profiling results [38], not solely relying on clinicians’ knowledge of local and available clinical trials. Some profiling services provide these trial matching services, such as Foundation Medicine [39], though to date it is unclear how comprehensive or relevant these suggestions are for patients or how often these suggestions are implemented. In addition, clinical trial slots for dose escalation studies are intermittently available or rapidly fill for small dose escalation cohorts so it is important to be able to capture this rapidly altering data. It is important to note that the development of algorithm-based systems can take initial significant investment [40].
Unfortunately, information on accessing off-licence treatment is unavailable, including how often drug applications are accepted or rejected.
Patients clinically deteriorated
One of the best-reported outcomes was the clinical deterioration of patients, whether that was declining performance status, admission to hospice care or death. By the nature of MTBs patients that are considered are often late stage, have rare cancers or poor prognosis. As a result, it is inevitable that they may decline during the process. However, accelerating the process of review will inevitably improve the chances of patients reviewed by MTBs, such as moving to liquid biopsies to reduce wait times for tissue acquisition and preparation or engaging with community teams that refer to the MTBs to facilitate earlier and more accurate referrals, thus decreasing the need for pre-sequencing MTB reviews to evaluate suitability for genomic testing.
Standardised reporting
In stark contrast to the criteria for publishing on clinical trials [41], there was no standard reporting for MTBs. This is evidenced by the difficulty in obtaining and analysing the data from this review due to the number of missing values. It is important to understand the reasoning behind patient attrition to be able to improve processes and understand barriers to access, but also to gain an accurate picture of patients that benefit from targeted therapy. Therefore, we suggest a broader community-level discussion on standardised reporting in MTBs. In addition, providing standard reporting for researchers allow for accurate contrast of approaches, whether that is process or testing driven.
Aiming for a systematic and continuous assessment of attrition and opportunities for therapeutic evolution, we suggest the following categories as a minimum set; tissue type; testing performed; number and types of genetic changes included; variant allele frequency threshold; genomic scale used; the number of patients registered to MTBs; the number of patients with actionable mutations; attrition numbers and reasoning; the total number of patients accessing treatment based on MTB review; and turnaround times from tissue acquisition to discussion at MTB.
Conclusions
Attrition within MTBs is a pervasive issue that is experienced globally, and as omics-derived data continues to increase alongside new targeted therapies and a growing literature base it is more important than ever to integrate new technologies to guide and aid clinicians in decision-making. Consistent reporting is important to understand barriers to accessing treatment via MTBs, and more work needs to be done to understand how often patients are unable to access off-licence treatment and clinical trials.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Wheeler DA, Wang L. From human genome to cancer genome: the first decade. Genome Res. 2013;23:1054–62.
Xuan J, Yu Y, Qing T, Guo L, Shi L. Next-generation sequencing in the clinic: promises and challenges. Cancer Lett. 2013;340:284–95.
Morganti S, Tarantino P, Ferraro E, D’Amico P, Viale G, Trapani D, et al. Complexity of genome sequencing and reporting: next generation sequencing (NGS) technologies and implementation of precision medicine in real life. Crit Rev Oncol Hematol. 2019;133:171–82.
Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff. 2018;37:694–701.
NIH. Targeted Therapy to Treat Cancer. 2020 [cited October 1, 2021]. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies
Normanno N, Barberis M, De Marinis F, Gridelli C. Molecular and genomic profiling of lung cancer in the era of precision medicine: a position paper from the Italian Association of Thoracic Oncology (AIOT). Cancers. 2020;12:1627.
Blackhall F, Cappuzzo F. Crizotinib: from discovery to accelerated development to front-line treatment. Ann Oncol. 2016;27:iii35–41.
Drilon A, Clark JW, Weiss J, Ou S-HI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26:47–51.
Tanizaki J, Okamoto I, Okamoto K, Takezawa K, Kuwata K, Yamaguchi H, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6:1624–31.
van de Haar J, Hoes L, Voest E. Advancing molecular tumour boards: highly needed to maximise the impact of precision medicine. ESMO Open. 2019;4:e000516.
Kato S, Kim KH, Lim HJ, Boichard A, Nikanjam M, Weihe E, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun. 2020;11:4965.
Larson KL, Huang B, Weiss HL, Hull P, Westgate PM, Miller RW, et al. Clinical outcomes of molecular tumor boards: a systematic review. JCO Precis Oncol. 2021;5:1122–32.
Ghazani AA, Oliver NM, St Pierre JP, Garofalo A, Rainville IR, Hiller E, et al. Assigning clinical meaning to somatic and germ-line whole-exome sequencing data in a prospective cancer precision medicine study. Genet Med. 2017;19:787–95.
Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–91.
Alix-Panabières C. The future of liquid biopsy. Nature. 2020;579:S9.
Rothwell DG, Ayub M, Cook N, Thistlethwaite F, Carter L, Dean E, et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat Med. 2019;25:738–43.
Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25:744–50. https://doi.org/10.1038/s41591-019-0407-5.
Bohan SS, Sicklick JK, Kato S, Okamura R, Miller VA, Leyland-Jones B, et al. Attrition of patients on a precision oncology trial: analysis of the I-PREDICT experience. Oncologist. 2020;25:e1803–6.
Choi IS, Kato S, Fanta PT, Leichman L, Okamura R, Raymond VM, et al. Genomic profiling of blood-derived circulating tumor DNA from patients with colorectal cancer: implications for response and resistance to targeted therapeutics. Mol Cancer Ther. 2019;18:1852–62.
Thakral D, Gupta R, Sahoo RK, Verma P, Kumar I, Vashishtha S. Real-time molecular monitoring in acute myeloid leukemia with circulating tumor DNA. Front Cell Dev Biol. 2020;8:604391.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24–224ra24.
Yang Y-C, Wang D, Jin L, Yao H-W, Zhang J-H, Wang J, et al. Circulating tumor DNA detectable in early- and late-stage colorectal cancer patients. Biosci Rep. 2018;38. https://portlandpress.com/bioscirep/article/doi/10.1042/BSR20180322/57926/Circulating-tumor-DNA-detectable-in-early-and
Wu CC, Maher MM, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. Am J Roentgenol. 2011;196:W678–82.
Schütte M, Ogilvie LA, Rieke DT, Lange BMH, Yaspo M-L, Lehrach H. Cancer precision medicine: why more is more and DNA is not enough. Public Health Genomics. 2017;20:70–80.
Hurst CD, Knowles MA. Multi-omic profiling refines the molecular view. Nat Rev Clin Oncol. 2018;15:203–4.
Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9:77–102.
Kurnit KC, Dumbrava EEI, Litzenburger B, Khotskaya YB, Johnson AM, Yap TA, et al. Precision oncology decision support: current approaches and strategies for the future. Clin Cancer Res. 2018;24:2719–31.
Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov. 2020;19:583–4.
Toma M, Felisi M, Bonifazi D, Bonifazi F, Giannuzzi V, Reggiardo G, et al. Paediatric medicines in europe: the paediatric regulation—is it time for reform? Front Med. 2021;8. https://www.frontiersin.org/articles/10.3389/fmed.2021.593281/full
European Medicines Agency and European Commission (DG Health and Food Safety) action plan on paediatrics. Action Plan on Paediatrics. 2020 [cited August 18, 2021]. https://www.ema.europa.eu/en/documents/report/european-medicines-agency-european-commission-dg-health-food-safety-action-plan-paediatrics_en.pdf
FDA. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. 2020 [cited August 13, 2021]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial
Al-Baimani K, Jonker H, Zhang T, Goss GD, Laurie SA, Nicholas G, et al. Are clinical trial eligibility criteria an accurate reflection of a real-world population of advanced non-small-cell lung cancer patients? Curr Oncol. 2018;25:291–7.
Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals. J Am Med Assoc. 2007;297:1233.
Liu R, Rizzo S, Whipple S, Pal N, Pineda AL, Lu M, et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592:629–33.
Kim ES, Bernstein D, Hilsenbeck SG, Chung CH, Dicker AP, Ersek JL, et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol. 2015;33:2815–20.
Kim ES, Bruinooge SS, Roberts S, Ison G, Lin NU, Gore L, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35:3737–44.
Patel NM, Michelini VV, Snell JM, Balu S, Hoyle AP, Parker JS, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23:179–85.
Alexander M, Solomon B, Ball DL, Sheerin M, Dankwa-Mullan I, Preininger AM, et al. Evaluation of an artificial intelligence clinical trial matching system in Australian lung cancer patients. JAMIA Open. 2020;3:209–15.
Foundation Medicine. Clinical Research & Trial Matching. 2021 [cited October 25, 2021]. https://www.foundationmedicine.com/service/clinical-research-and-trial-matching
Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2019;33:10–18.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18.
Funding
This work was supported by the AstraZeneca iDECIDE Programme (grant no. 119106) awarded to Manchester Cancer Research Centre and Cancer Research UK (CRUK), Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fundacion Científica—Asociacion Espanola Contra el Cancer (FC -AECC), via an Accelerator Award [A29374] through the CRUK Manchester Institute [C147/A25254]. The role of the funder: This review was not registered and a protocol was not prepared.
Author information
Authors and Affiliations
Contributions
HF—conceptulalization, data curation, formal analysis, investigation, project administration and writing—original draft. DMG—conceptulalization, supervision and writing—review and editing. LC—conceptulalization, supervision and writing—review and editing. PO’R—conceptulalization, formal analysis and writing—review and editing. DL—conceptulalization, funding acquisition, supervision and writing —review and editing. AF—conceptulalization, supervision and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
This work is funded in part through a grant awarded by AstraZeneca. Hannah Frost, Donna M. Graham and Louise Carter are all authors of a paper used in this review. Dónal Landers is a director of DeLondra Oncology Ltd., which provides Pharmaceutical Medicine consultancy to Astrazeneca Ltd and Athenex plc.
Ethics approval and consent to participate
As this work is based on searches of the published literature, no additional ethics approval was required.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frost, H., Graham, D.M., Carter, L. et al. Patient attrition in Molecular Tumour Boards: a systematic review. Br J Cancer 127, 1557–1564 (2022). https://doi.org/10.1038/s41416-022-01922-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01922-3