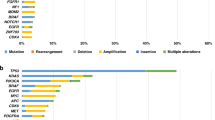
Abstract
Over the past 15 years, rapid progress has been made in developmental therapeutics, especially regarding the use of matched targeted therapies against specific oncogenic molecular alterations across cancer types. Molecular tumour boards (MTBs) are panels of expert physicians, scientists, health-care providers and patient advocates who review and interpret molecular-profiling results for individual patients with cancer and match each patient to available therapies, which can include investigational drugs. Interpretation of the molecular alterations found in each patient is a complicated task that requires an understanding of their contextual functional effects and their correlations with sensitivity or resistance to specific treatments. The criteria for determining the actionability of molecular alterations and selecting matched treatments are constantly evolving. Therefore, MTBs have an increasingly necessary role in optimizing the allocation of biomarker-directed therapies and the implementation of precision oncology. Ultimately, increased MTB availability, accessibility and performance are likely to improve patient care. The challenges faced by MTBs are increasing, owing to the plethora of identifiable molecular alterations and immune markers in tumours of individual patients and their evolving clinical significance as more and more data on patient outcomes and results from clinical trials become available. Beyond next-generation sequencing, broader biomarker analyses can provide useful information. However, greater funding, resources and expertise are needed to ensure the sustainability of MTBs and expand their outreach to underserved populations. Harmonization between practice and policy will be required to optimally implement precision oncology. Herein, we discuss the evolving role of MTBs and current and future considerations for their use in precision oncology.
Key points
-
In the era of precision oncology, molecular tumour boards (MTBs) have an increasingly important role in optimizing treatment selection to improve outcomes by reviewing and interpreting molecular-profiling data and matching patients with appropriate available molecularly targeted therapies, which can include investigational drugs.
-
Indeed, MTB review of the interpretation of molecular alterations is crucially important for identifying matched treatment options that target the activity of the altered genes directly or within their respective established actionable genomic pathways.
-
Frequently updated criteria for the actionability of molecular alterations are essential to select and prioritize treatments, taking into consideration the level of evidence, although treatment outcomes depend on access to matched therapies and/or clinical trials.
-
Certain precision oncology trials include MTB review in routine practice; however, despite the reported clinical benefits of matched therapy, few patients can access the relevant therapies in a timely manner.
-
Standardization of and consensus regarding MTB implementation and quality requirements, novel digital platforms for data interpretation and annotation, expanded access to medications and evidence of therapeutic actionability from real-world data repositories and prospective biomarker validation studies would provide increased potential to improve patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
MedlinePlus. What is the difference between precision medicine and personalized medicine? What about pharmacogenomics? https://medlineplus.gov/genetics/understanding/precisionmedicine/precisionvspersonalized/ (2022).
Tsimberidou, A. M. et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): an MD Anderson Precision Medicine Study. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00002 (2017).
Christofyllakis, K. et al. Cost-effectiveness of precision cancer medicine — current challenges in the use of next generation sequencing for comprehensive tumour genomic profiling and the role of clinical utility frameworks (Review). Mol. Clin. Oncol. 16, 21 (2022).
Tsimberidou, A. M. et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin. Cancer Res. 18, 6373–6383 (2012).
Von Hoff, D. D. et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 28, 4877–4883 (2010).
Tsimberidou, A. M. et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin. Cancer Res. 20, 4827–4836 (2014).
Tsimberidou, A. M. et al. Long-term overall survival and prognostic score predicting survival: the IMPACT study in precision medicine. J. Hematol. Oncol. 12, 145 (2019).
Larson, K. L. et al. Clinical outcomes of molecular tumor boards: a systematic review. JCO Precis. Oncol. https://doi.org/10.1200/PO.20.00495 (2021).
Luthra, R. et al. A targeted high-throughput next-generation sequencing panel for clinical screening of mutations, gene amplifications, and fusions in solid tumors. J. Mol. Diagn. 19, 255–264 (2017).
Lih, C. J. et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: molecular analysis for therapy choice clinical trial. J. Mol. Diagn. 19, 313–327 (2017).
Goswami, R. S. et al. Identification of factors affecting the success of next-generation sequencing testing in solid tumors. Am. J. Clin. Pathol. 145, 222–237 (2016).
Ramani, N. S. et al. Factors impacting clinically relevant RNA fusion assays using next-generation sequencing. Arch. Pathol. Lab. Med. 145, 1405–1412 (2021).
Jennings, L. J. et al. Guidelines for validation of next-generation sequencing-based oncology panels: a Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 19, 341–365 (2017).
Li, M. M. et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 19, 4–23 (2017).
Foundation Medicine. FoundationOne CDx. https://www.foundationmedicine.com/test/foundationone-cdx (2023).
TEMPUS. Genomic Profiling. https://www.tempus.com/oncology/genomic-profiling/xt-xr/ (2023).
CARIS LIFE SCIENCES. Molecular Profiling. https://www.carislifesciences.com/products-and-services/molecular-profiling/testing-menu/ (2023).
Malone, E. R., Oliva, M., Sabatini, P. J. B., Stockley, T. L. & Siu, L. L. Molecular profiling for precision cancer therapies. Genome Med. 12, 8 (2020).
Galuppini, F. et al. Tumor mutation burden: from comprehensive mutational screening to the clinic. Cancer Cell Int. 19, 209 (2019).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
U.S. Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (2020).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Gelsomino, F., Barbolini, M., Spallanzani, A., Pugliese, G. & Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat. Rev. 51, 19–26 (2016).
Boland, C. R. & Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 138, 2073–2087.e3 (2010).
Le, D. T. et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 38, 11–19 (2020).
Le, D. T. et al. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: final analysis of KEYNOTE-164. Eur. J. Cancer 186, 185–195 (2023).
O’Malley, D. M. et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J. Clin. Oncol. 40, 752–761 (2022).
Maio, M. et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 33, 929–938 (2022).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Yoshino, T. et al. Pembrolizumab in Asian patients with microsatellite-instability-high/mismatch-repair-deficient colorectal cancer. Cancer Sci. 114, 1026–1036 (2023).
Oaknin, A. et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer 10, e003777 (2022).
Oaknin, A. et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 6, 1766–1772 (2020).
Oaknin, A. et al. Safety, efficacy, and biomarker analyses of dostarlimab in patients with endometrial cancer: interim results of the phase I GARNET study. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-22-3915 (2023).
Lenz, H. J. et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkmate 142 study. J. Clin. Oncol. 40, 161–170 (2022).
FoundationOne CDx. Technical Information F1CDx. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S029C.pdf (2020).
U.S. Food and Drug Administration. VENTANA: Ventana Anti-MLH1 (M1) Mouse Monoclonal Primary Antibody. https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210001D.pdf (2022).
U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/site Agnostic Indication. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (2017).
MERCK. FDA Converts to Full Approval Indication for KEYTRUDA® (Pembrolizumab) for Certain Adult and Pediatric Patients With Advanced Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Solid Tumors. https://www.merck.com/news/fda-converts-to-full-approval-indication-for-keytruda-pembrolizumab-for-certain-adult-and-pediatric-patients-with-advanced-microsatellite-instability-high-msi-h-or-mismatch-repair-deficient/ (2023).
U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Dostarlimab-gxly for dMMR Advanced Solid Tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (2023).
Lebofsky, R. et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol. Oncol. 9, 783–790 (2015).
Normanno, N., Cervantes, A., Ciardiello, F., De Luca, A. & Pinto, C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat. Rev. 70, 1–8 (2018).
Zill, O. A. et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin. Cancer Res. 24, 3528–3538 (2018).
Polivka, J. Jr., Pesta, M. & Janku, F. Testing for oncogenic molecular aberrations in cell-free DNA-based liquid biopsies in the clinic: are we there yet. Expert Rev. Mol. Diagn. 15, 1631–1644 (2015).
Fleischhacker, M. & Schmidt, B. Circulating nucleic acids (CNAs) and cancer — a survey. Biochim. Biophys. Acta 1775, 181–232 (2007).
Suraj, S., Dhar, C. & Srivastava, S. Circulating nucleic acids: an analysis of their occurrence in malignancies. Biomed. Rep. 6, 8–14 (2017).
Janku, F. et al. Development and validation of an ultradeep next-generation sequencing assay for testing of plasma cell-free DNA from patients with advanced cancer. Clin. Cancer Res. 23, 5648–5656 (2017).
Said, R., Guibert, N., Oxnard, G. R. & Tsimberidou, A. M. Circulating tumor DNA analysis in the era of precision oncology. Oncotarget 11, 188–211 (2020).
Sanchez, N. S. et al. Identification of actionable genomic alterations using circulating cell-free DNA. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00017 (2019).
Laufer-Geva, S. et al. The clinical impact of comprehensive genomic testing of circulating cell-free DNA in advanced lung cancer. J. Thorac. Oncol. 13, 1705–1716 (2018).
TEMPUS | ONCOLOGY. xF+ Gene List. https://www.tempus.com/wp-content/uploads/2022/12/Tempus-xFPlus_Gene-Panel.pdf (2022).
FoundationOne Liquid CDx. Technical Specifications. https://assets.ctfassets.net/w98cd481qyp0/wVEm7VtICYR0sT5C1VbU7/fd055e0476183a6acd4eae6b583e3a00/F1LCDx_Technical_Specs_072021.pdf (2021).
Guardant Complete 360. An Expanded Panel of Biomarkers. https://www.guardantcomplete.com/guardant-portfolio/360 (2021).
U.S. Food and Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools (2023).
CISION PR Newswire. Guardant Health Receives Regulatory Approval for Guardant360 CDx in Japan. https://www.prnewswire.com/news-releases/guardant-health-receives-regulatory-approval-for-guardant360-cdx-in-japan-301501649.html (2022).
FoundationOne Medicine. Clinical Development & CDx. https://www.foundationmedicine.com/service/clinical-development-and-cdx (2022).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Coombs, C. C. et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin. Cancer Res. 24, 5918–5924 (2018).
Bauml, J. & Levy, B. Clonal hematopoiesis: a new layer in the liquid biopsy story in lung cancer. Clin. Cancer Res. 24, 4352–4354 (2018).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic — implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Jensen, K. et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 7, 107–110 (2021).
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 36, 1631–1641 (2018).
Coombs, C. C. et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21, 374–382.e4 (2017).
Chan, H. T., Chin, Y. M., Nakamura, Y. & Low, S. K. Clonal hematopoiesis in liquid biopsy: from biological noise to valuable clinical implications. Cancers 12, 2277 (2020).
Razavi, P. et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 25, 1928–1937 (2019).
National Cancer Institute. Germline Mutation. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/germline-mutation (2023).
Moore, K. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379, 2495–2505 (2018).
DiSilvestro, P. et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J. Clin. Oncol. 41, 609–617 (2023).
Banerjee, S. et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 22, 1721–1731 (2021).
Pujade-Lauraine, E. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1274–1284 (2017).
Poveda, A. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 22, 620–631 (2021).
Tutt, A. N. J. et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med. 384, 2394–2405 (2021).
Yamauchi, H. et al. Adjuvant olaparib in the subset of patients from Japan with BRCA1- or BRCA2-mutated high-risk early breast cancer from the phase 3 OlympiA trial. Breast Cancer 30, 596–605 (2023).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377, 523–533 (2017).
Robson, M. E. et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 30, 558–566 (2019).
Im, S. A. et al. Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis. Sci. Rep. 10, 8753 (2020).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Golan, T. et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 381, 317–327 (2019).
Kindler, H. L. et al. Overall survival results from the POLO trial: a phase III study of active maintenance olaparib versus placebo for germline BRCA-mutated metastatic pancreatic cancer. J. Clin. Oncol. 40, 3929–3939 (2022).
Hussain, M. et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 383, 2345–2357 (2020).
Matsubara, N. et al. Olaparib efficacy in patients with metastatic castration-resistant prostate cancer and BRCA1, BRCA2, or ATM alterations identified by testing circulating tumor DNA. Clin. Cancer Res. 29, 92–99 (2023).
Matsubara, N. et al. Olaparib in patients with mCRPC with homologous recombination repair gene alterations: PROfound Asian subset analysis. Jpn. J. Clin. Oncol. 52, 441–448 (2022).
Mirza, M. R. et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 375, 2154–2164 (2016).
Del Campo, J. M. et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J. Clin. Oncol. 37, 2968–2973 (2019).
Coleman, R. L. et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 1949–1961 (2017).
O’Malley, D. M. et al. Clinical and molecular characteristics of ARIEL3 patients who derived exceptional benefit from rucaparib maintenance treatment for high-grade ovarian carcinoma. Gynecol. Oncol. 167, 404–413 (2022).
Ledermann, J. A. et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 21, 710–722 (2020).
Abida, W. et al. Rucaparib for the treatment of metastatic castration-resistant prostate cancer associated with a DNA damage repair gene alteration: final results from the phase 2 TRITON2 study. Eur. Urol. 84, 321–330 (2023).
Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379, 753–763 (2018).
Chan, H. T. et al. Clinical significance of clonal hematopoiesis in the interpretation of blood liquid biopsy. Mol. Oncol. 14, 1719–1730 (2020).
Koch, A. et al. Analysis of DNA methylation in cancer: location revisited. Nat. Rev. Clin. Oncol. 15, 459–466 (2018).
Xu, L. et al. Long-read sequencing identifies novel structural variations in colorectal cancer. PLoS Genet. 19, e1010514 (2023).
Fisher, C. L., Dillon, R., Anguita, E., Morris-Rosendahl, D. J. & Awan, A. R. A novel bead-capture nanopore sequencing method for large structural rearrangement detection in cancer. J. Mol. Diagn. 24, 1264–1278 (2022).
Chen, Y. et al. Gene fusion detection and characterization in long-read cancer transcriptome sequencing data with FusionSeeker. Cancer Res. 83, 28–33 (2023).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
U.S. Food and Drug Administration. VITRAKVI: Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210861s008lbl.pdf (2022).
U.S. Food and Drug Administration. ROZLYTREK: Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212725s006lbl.pdf (2022).
von Bubnoff, N., Schneller, F., Peschel, C. & Duyster, J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet 359, 487–491 (2002).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880 (2001).
Goyal, L. et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 7, 252–263 (2017).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl. Med. 4, 120ra117 (2012).
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA 105, 2070–2075 (2008).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Yu, H. A. et al. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin. Cancer Res. 24, 3108–3118 (2018).
Kong, L. L. et al. Structural pharmacological studies on EGFR T790M/C797S. Biochem. Biophys. Res. Commun. 488, 266–272 (2017).
Uchibori, K. et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat. Commun. 8, 14768 (2017).
Niederst, M. J. et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin. Cancer Res. 21, 3924–3933 (2015).
Yu, H. A. et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 1, 982–984 (2015).
Thress, K. S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 21, 560–562 (2015).
Song, H. N. et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small cell lung cancer. J. Thorac. Oncol. 11, e45–47 (2016).
Weigelt, B. et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin. Cancer Res. 23, 6708–6720 (2017).
Pettitt, S. J. et al. Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discov. 10, 1475–1488 (2020).
Lin, K. K. et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 9, 210–219 (2019).
Shroff, R. T. et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis. Oncol. 2018, 00316 (2018).
Afghahi, A. et al. Tumor BRCA1 reversion mutation arising during neoadjuvant platinum-based chemotherapy in triple-negative breast cancer is associated with therapy resistance. Clin. Cancer Res. 23, 3365–3370 (2017).
Tobalina, L., Armenia, J., Irving, E., O’Connor, M. J. & Forment, J. V. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann. Oncol. 32, 103–112 (2021).
Zong, H. et al. Comprehensive analysis of somatic reversion mutations in homologous recombination repair (HRR) genes in a large cohort of Chinese pan-cancer patients. J. Cancer 13, 1119–1129 (2022).
Hilton, J. et al. Results of the phase I CCTG IND.231 trial of CX-5461 in patients with advanced solid tumors enriched for DNA-repair deficiencies. Nat. Commun. 13, 3607 (2022).
U.S. Food and Drug Administration. Erbitux: Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125084s279lbl.pdf (2021).
U.S. Food and Drug Administration. Vectibix: Highlights of Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125147s210lbl.pdf (2021).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007).
Leonetti, A. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 121, 725–737 (2019).
National Comprehensive Cancer Network. NCCN Guidelines. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (2023).
Tsimberidou, A. M. et al. Phase 1 study of M2698, a p70S6K/AKT dual inhibitor, in patients with advanced cancer. J. Hematol. Oncol. 14, 127 (2021).
Meric-Bernstam, F. et al. A decision support framework for genomically informed investigational cancer therapy. J. Natl Cancer Inst. 107, djv098 (2015).
Johnson, A. et al. Clinical use of precision oncology decision support. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00036 (2017).
Kurnit, K. C. et al. ‘Personalized cancer therapy’: a publicly available precision oncology resource. Cancer Res. 77, e123–e126 (2017).
Zeng, J. et al. OCTANE: oncology clinical trial annotation engine. JCO Clin. Cancer Inf. 3, 1–11 (2019).
Zeng, J. et al. Operationalization of next-generation sequencing and decision support for precision oncology. JCO Clin. Cancer Inf. 3, 1–12 (2019).
Mangat, P. K. et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) study. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00122 (2018).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00011 (2017).
Patterson, S. E., Statz, C. M., Yin, T. & Mockus, S. M. Utility of the JAX Clinical Knowledgebase in capture and assessment of complex genomic cancer data. npj Precis. Oncol. 3, 2 (2019).
Tamborero, D. et al. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat. Cancer 3, 251–261 (2022).
Johnson, A. et al. The right drugs at the right time for the right patient: the MD Anderson precision oncology decision support platform. Drug Discov. Today 20, 1433–1438 (2015).
Flaherty, K. T. & Conley, B. A. Molecular Analysis for Therapy Choice (NCI-MATCH): A Precision Medicine Signal-Seeking Trial in Oncology. https://www.personalizedmedonc.com/articles/3443:molecular-analysis-for-therapy-choice-nci-match (2023).
Van Allen, E. M. et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat. Med. 20, 682–688 (2014).
Andre, F. et al. Prioritizing targets for precision cancer medicine. Ann. Oncol. 25, 2295–2303 (2014).
Mateo, J. et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). Ann. Oncol. 29, 1895–1902 (2018).
Leichsenring, J. et al. Variant classification in precision oncology. Int. J. Cancer 145, 2996–3010 (2019).
U.S. Food and Drug Administration. CDRH’S Approach to Tumor Profiling Next Generation Sequencing Tests. https://www.fda.gov/media/109050/download (2023).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019).
Jain, N. M., Culley, A., Micheel, C. M., Osterman, T. J. & Levy, M. A. Learnings from precision clinical trial matching for oncology patients who received NGS testing. JCO Clin. Cancer Inf. 5, 231–238 (2021).
Meropol, N. J. et al. Barriers to clinical trial participation as perceived by oncologists and patients. J. Natl Compr. Cancer Netw. 5, 655–664 (2007).
Mills, E. J. et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 7, 141–148 (2006).
St Germain, D. C. & McCaskill-Stevens, W. Use of a clinical trial screening tool to enhance patient accrual. Cancer 127, 1630–1637 (2021).
Unger, J. M., Cook, E., Tai, E. & Bleyer, A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am. Soc. Clin. Oncol. Educ. Book. 35, 185–198 (2016).
Unger, J. M. et al. ‘When offered to participate’: a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J. Natl Cancer Inst. 113, 244–257 (2021).
U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Dabrafenib in Combination with Trametinib for Unresectable or Metastatic Solid Tumors with BRAF V600E Mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (2022).
European Medicines Agency. Mekinist. https://www.ema.europa.eu/en/medicines/human/EPAR/mekinist (2022).
European Medicines Agency. Tafinlar. https://www.ema.europa.eu/en/medicines/human/EPAR/tafinlar (2021).
Fountzilas, E., Said, R. & Tsimberidou, A. M. Expanded access to investigational drugs: balancing patient safety with potential therapeutic benefits. Expert Opin. Investig. Drugs 27, 155–162 (2018).
Hull, R. et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 20, 2058–2074 (2020).
Knaul, F. M., Bhadelia, A., Atun, R. & Frenk, J. Achieving effective universal health coverage and diagonal approaches to care for chronic illnesses. Health Aff. 34, 1514–1522 (2015).
Bharadwaj, M., Vallurupalli, M. & Huang, F. W. Global precision oncology: a call to action on expanding access to targeted cancer therapies. Oncologist 26, 353–355 (2021).
Nelson, A. M., Milner, D. A., Rebbeck, T. R. & Iliyasu, Y. Oncologic care and pathology resources in Africa: survey and recommendations. J. Clin. Oncol. 34, 20–26 (2016).
Lopez, M. S. et al. Cervical cancer prevention and treatment in Latin America. J. Surg. Oncol. 115, 615–618 (2017).
Brandao, M. et al. Survival impact and cost–effectiveness of a multidisciplinary tumor board for breast cancer in Mozambique, Sub-Saharan Africa. Oncologist 26, e996–e1008 (2021).
Incorvaia, L., Russo, A. & Cinieri, S. The molecular tumor board: a tool for the governance of precision oncology in the real world. Tumori 108, 288–290 (2022).
Erdmann, J. All aboard: will molecular tumor boards help cancer patients? Nat. Med. 21, 655–656 (2015).
Mosele, F. et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 31, 1491–1505 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Alva, A. S. et al. Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 39, 2443–2451 (2021).
Ahn, E. R. et al. Palbociclib in patients with non-small-cell lung cancer with CDKN2A alterations: results from the targeted agent and profiling utilization registry study. JCO Precis. Oncol. 4, 757–766 (2020).
Song, I. W. et al. Precision oncology: evolving clinical trials across tumor types. Cancers 15, 1967 (2023).
Vo, H. H. et al. Challenges and opportunities associated with the MD Anderson IMPACT2 randomized study in precision oncology. npj Precis. Oncol. 6, 78 (2022).
Tsimberidou, A. M. et al. Precision medicine: preliminary results from the Initiative for Molecular Profiling and Advanced Cancer Therapy 2 (IMPACT2) study. npj Precis. Oncol. 5, 21 (2021).
Tredan, O. et al. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: analysis from the ProfiLER trial. Ann. Oncol. 30, 757–765 (2019).
Bonneville-Levard, A. et al. Molecular profile to guide personalized medicine in adult patients with primary brain tumors: results from the ProfiLER trial. Med. Oncol. 39, 4 (2021).
Varnier, R. et al. Actionable molecular alterations in advanced gynaecologic malignancies: updated results from the ProfiLER programme. Eur. J. Cancer 118, 156–165 (2019).
Tredan O, P. D. et al. Increasing targeted therapy options for patients with relapsed cancer with broader somatic gene panel analysis from the primary tumor: the Profiler02 randomized phase II trial. J. Clin. Oncol. 40, 1104659 (2022).
ECOG-ACRIN Cancer Research Group. NCI-MATCH Searchable Treatment Arms Table. https://ecog-acrin.org/nci-match-eay131/ (2022).
Flaherty, K. T. et al. The molecular analysis for therapy choice (NCI-MATCH) trial: lessons for genomic trial design. J. Natl Cancer Inst. 112, 1021–1029 (2020).
Flaherty, K. T. et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 38, 3883–3894 (2020).
Basse, C. et al. Relevance of a molecular tumour board (MTB) for patients’ enrolment in clinical trials: experience of the Institut Curie. ESMO Open. 3, e000339 (2018).
Tao, J. J. et al. Real-world outcomes of an automated physician support system for genome-driven oncology. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00066 (2019).
Pagel, K. A. et al. Integrated informatics analysis of cancer-related variants. JCO Clin. Cancer Inf. 4, 310–317 (2020).
Reardon, B. et al. Integrating molecular profiles into clinical frameworks through the molecular oncology almanac to prospectively guide precision oncology. Nat. Cancer 2, 1102–1112 (2021).
Farhangfar, C. J. et al. Impact of a clinical genomics program on trial accrual for targeted treatments: practical approach overcoming barriers to accrual for underserved patients. JCO Clin. Cancer Inf. 6, e2200011 (2022).
Kato, S. et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-one strategy. Nat. Commun. 11, 4965 (2020).
Rodon, J. et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 25, 751–758 (2019).
Lamping, M. et al. Support of a molecular tumour board by an evidence-based decision management system for precision oncology. Eur. J. Cancer 127, 41–51 (2020).
Moore, D. A. et al. Prospective analysis of 895 patients on a UK Genomics Review Board. ESMO Open 4, e000469 (2019).
Rothwell, D. G. et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat. Med. 25, 738–743 (2019).
Righi, M. L. V., Martinez, P., Silva, A., Umpierrez, C. & Rodriguez, R. Oncotherapy: a decision support system to validate oncological treatments. Stud. Health Technol. Inf. 264, 1799–1800 (2019).
Tamborero, D. et al. Support systems to guide clinical decision-making in precision oncology: the Cancer Core Europe Molecular Tumor Board Portal. Nat. Med. 26, 992–994 (2020).
Andre, F. et al. Genomics to select treatment for patients with metastatic breast cancer. Nature 610, 343–348 (2022).
Sadik, H. et al. Impact of clinical practice gaps on the implementation of personalized medicine in advanced non-small-cell lung cancer. JCO Precis. Oncol. 6, e2200246 (2022).
Kim, E. S. et al. Continuing to broaden eligibility criteria to make clinical trials more representative and inclusive: ASCO-Friends of Cancer Research Joint Research Statement. Clin. Cancer Res. 27, 2394–2399 (2021).
Love, T. M., Anaya, D. A., Prime, M. S., Ardolino, L. & Ekinci, O. Development and validation of ACTE-MTB: a tool to systematically assess the maturity of molecular tumor boards. PLoS ONE 17, e0268477 (2022).
Zeng, J. & Shufean, M. A. Molecular-based precision oncology clinical decision making augmented by artificial intelligence. Emerg. Top. Life Sci. 5, 757–764 (2021).
Ballester, P. J. & Carmona, J. Artificial intelligence for the next generation of precision oncology. npj Precis. Oncol. 5, 79 (2021).
Azuaje, F. Artificial intelligence for precision oncology: beyond patient stratification. npj Precis. Oncol. 3, 6 (2019).
Bhalla, S. & Lagana, A. Artificial intelligence for precision oncology. Adv. Exp. Med. Biol. 1361, 249–268 (2022).
Hernandez-Boussard, T. et al. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat. Med. 27, 2065–2066 (2021).
Bodenmiller, B. Multiplexed epitope-based tissue imaging for discovery and healthcare applications. Cell Syst. 2, 225–238 (2016).
Mateo, J. et al. Delivering precision oncology to patients with cancer. Nat. Med. 28, 658–665 (2022).
O’Regan, P. et al. Digital ECMT cancer trial matching tool: an open source research application to support oncologists in the identification of precision medicine clinical trials. JCO Clin. Cancer Inf. 7, e2200137 (2023).
Xu, Q. et al. OncoPubMiner: a platform for mining oncology publications. Brief. Bioinform. 23, bbac383 (2022).
Bosserman, L. D. et al. Pyramidal decision support framework leverages subspecialty expertise across enterprise to achieve superior cancer outcomes and personalized, precision care plans. J. Clin. Med. 11, 6738 (2022).
Kurz, N. S. et al. Identifying actionable variants in cancer — the dual web and batch processing tool MTB-Report. Stud. Health Technol. Inf. 296, 73–80 (2022).
de Andrade, K. C. et al. The TP53 database: transition from the International Agency for Research on Cancer to the US National Cancer Institute. Cell Death Differ. 29, 1071–1073 (2022).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Chunn, L. M. et al. Mastermind: a comprehensive genomic association search engine for empirical evidence curation and genetic variant interpretation. Front. Genet. 11, 577152 (2020).
Xu, Q. et al. OncoPDSS: an evidence-based clinical decision support system for oncology pharmacotherapy at the individual level. BMC Cancer 20, 740 (2020).
Gudmundsson, S. et al. Variant interpretation using population databases: lessons from gnomAD. Hum. Mutat. 43, 1012–1030 (2022).
Cline, M. S. et al. BRCA challenge: BRCA exchange as a global resource for variants in BRCA1 and BRCA2. PLoS Genet. 14, e1007752 (2018).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Patterson, S. E. et al. The clinical trial landscape in oncology and connectivity of somatic mutational profiles to targeted therapies. Hum. Genomics 10, 4 (2016).
The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Bayle, A. et al. Clinical utility of circulating tumor DNA sequencing with a large panel: a National Center for Precision Medicine (PRISM) study. Ann. Oncol. 34, 389–396 (2023).
Hlevnjak, M. et al. CATCH: a prospective precision oncology trial in metastatic breast cancer. JCO Precis. Oncol. https://doi.org/10.1200/PO.20.00248 (2021).
Irmisch, A. et al. The tumor profiler study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell 39, 288–293 (2021).
Conley, B. A. & Doroshow, J. H. Molecular analysis for therapy choice: NCI MATCH. Semin. Oncol. 41, 297–299 (2014).
Keller, R. B. et al. Programmatic precision oncology decision support for patients with gastrointestinal cancer. JCO Precis. Oncol. 7, e2200342 (2023).
Cannon, T. L. et al. Patient attendance at molecular tumor board: a new means of shared decision making. Curr. Probl. Cancer 46, 100860 (2022).
Le Tourneau, C. et al. An approach to solving the complex clinicogenomic data landscape in precision oncology: learnings from the design of WAYFIND-R, a global precision oncology registry. JCO Precis. Oncol. 6, e2200019 (2022).
Heinrich, K. et al. Lessons learned: the first consecutive 1000 patients of the CCCMunich(LMU) Molecular Tumor Board. J. Cancer Res. Clin. Oncol. 149, 1905–1915 (2023).
Thouvenin, J. et al. PRECISION: the Belgian molecular profiling program of metastatic cancer for clinical decision and treatment assignment. ESMO Open. 7, 100524 (2022).
Park, K. H. et al. Genomic landscape and clinical utility in Korean advanced pan-cancer patients from prospective clinical sequencing: K-MASTER program. Cancer Discov. 12, 938–948 (2022).
Malani, D. et al. Implementing a functional precision medicine tumor board for acute myeloid leukemia. Cancer Discov. 12, 388–401 (2022).
Lin, F. P. et al. Criteria-based curation of a therapy-focused compendium to support treatment recommendations in precision oncology. npj Precis. Oncol. 5, 58 (2021).
Jain, N. M. et al. Framework for implementing and tracking a molecular tumor board at a National Cancer Institute-Designated Comprehensive Cancer Center. Oncologist 26, e1962–e1970 (2021).
Acknowledgements
The work of the authors is supported in part by funds given generously to the Sheikh Khalifa al Nahyan Ben Zayed Institute of Personalized Cancer Therapy (to The University of Texas MD Anderson Cancer Center) and by The Bosarge Family Foundation (to M.K., A.J. and F.M.-B). The work of the authors is also supported in part by the NIH National Center for Advancing Translational Sciences grants UL1 TR000371 and 1U01 CA180964 (to F.M.-B.) and the NIH National Cancer Institute award number P30 CA016672 (to The University of Texas MD Anderson Cancer Center). A.M.T. acknowledges funding support from the Mr and Mrs Steven McKenzie’s Endowment and donor funds from Jamie’s Hope and Mrs and Mr Z.W. Arrott for The University of Texas MD Anderson Cancer Center Personalized Medicine Program (Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT)).
Author information
Authors and Affiliations
Contributions
A.M.T. and F.M.-B. contributed substantially to discussion of the content. All authors researched data for the article, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The University of Texas MD Anderson Cancer Center receives licensing fees for their Precision Oncology Decision Support (PODS) database from Philips Healthcare, which supports continued development of the PODS system. A.M.T. declares receipt of clinical trial research funding (to The University of Texas MD Anderson Cancer Center) from Agenus, IMMATICS, Novocure, OBI Pharma, Parker Institute for Cancer Immunotherapy, Tachyon, Tempus and Tvardi; fees for consulting or advisory roles for Avstera Therapeutics, Bioeclipse, BrYet, Diaccurate, Macrogenics, NEX-I and VinceRx; and travel expenses from ASCO, Cancer Care Crossroads, GenomeWeb Conference and Precision Medicine World Conference. F.M.-B. declares research funding (to The University of Texas MD Anderson Cancer Center) from Aileron Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi Sankyo, Debiopharm, eFFECTOR Therapeutics, Genentech, Guardant Health, Klus Pharma, Novartis, Puma Biotechnology, Taiho Pharmaceutical and Takeda Pharmaceuticals; consulting or advisory roles for AbbVie, Aduro BioTech, Alkermes, AstraZeneca, Daiichi Sankyo, DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman-La Roche, Genentech, GT Apeiron Therapeutics, Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini, OrigiMed, PACT Pharma, Parexel, Pfizer, Protai Bio, Samsung Bioepis, Seattle Genetics, Tallac Therapeutics, Tyra Biosciences, Xencor and Zymeworks; honoraria from Chugai Biopharmaceuticals; and other relationships with the European Organization for Research and Treatment of Cancer and European Society for Medical Oncology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C. Westphalen, who co-reviewed with V. Probst; J. Sicklick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BRCA Exchange: https://brcaexchange.org/
CKB: https://ckb.jax.org/
ClinicalTrials.gov: https://clinicaltrials.gov/
ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/
Digital ECMT cancer trial matching tool: https://trialmatch.digitalecmt.com/
gnomAD: https://gnomad.broadinstitute.org/
MASTERMIND: https://mastermind.genomenon.com/
MatchMiner: https://matchminer.org/
Model List of Essential Medicines: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
Molecular Oncology Almanac: https://moalmanac.org/
MTBP: https://www.mtbp.org/
MTB-Report: https://bioinformatics.umg.eu/research/projects/mtb-report/
OncoKB: https://www.oncokb.org/
OncoPDSS: https://oncopdss.capitalbiobigdata.com/
OncoPubMiner: https://oncopubminer.chosenmedinfo.com/lrSearch
OpenCRAVAT: https://opencravat.org/
Personalized Cancer Therapy Knowledge Base for Precision Oncology: https://pct.mdanderson.org/
PRISM: https://prism.center/
The TP53 Database: https://tp53.isb-cgc.org/
TOPOGRAPH: https://topograph.info/home.php
TuPro: https://eth-nexus.github.io/tu-pro_website/project/
Uniprot: https://www.uniprot.org/
WAYFIND-R: https://www.wayfind-r.com/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsimberidou, A.M., Kahle, M., Vo, H.H. et al. Molecular tumour boards — current and future considerations for precision oncology. Nat Rev Clin Oncol 20, 843–863 (2023). https://doi.org/10.1038/s41571-023-00824-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00824-4
This article is cited by
-
Personalisierte Medizin in der Onkologie
Die Pathologie (2024)
-
Molekulare Tumorboards
Forum (2024)
-
Registerbasierte Krebsforschung in Deutschland
Forum (2024)
-
Einsatz von Arzneimitteln außerhalb der Zulassung – Conditio sine qua non in der Präzisionsonkologie?
Forum (2024)