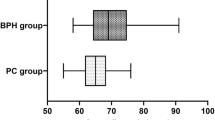
Abstract
Background
The objective of this study was to establish the contribution of PALB2 mutations to prostate cancer risk and to estimate survival among PALB2 carriers.
Methods
We genotyped 5472 unselected men with prostate cancer and 8016 controls for two Polish founder variants of PALB2 (c.509_510delGA and c.172_175delTTGT). In patients with prostate cancer, the survival of carriers of a PALB2 mutation was compared to that of non-carriers.
Results
A PALB2 mutation was found in 0.29% of cases and 0.21% of controls (odds ratio (OR) = 1.38; 95% confidence interval (CI) 0.70–2.73; p = 0.45). PALB2 mutation carriers were more commonly diagnosed with aggressive cancers of high (8–10) Gleason score than non-carriers (64.3 vs 18.1%, p < 0.0001). The OR for high-grade prostate cancer was 8.05 (95% CI 3.57–18.15, p < 0.0001). After a median follow-up of 102 months, the age-adjusted hazard ratio for all-cause mortality associated with a PALB2 mutation was 2.52 (95% CI 1.40–4.54; p = 0.0023). The actuarial 5-year survival was 42% for PALB2 carriers and was 72% for non-carriers (p = 0.006).
Conclusion
In Poland, PALB2 mutations predispose to an aggressive and lethal form of prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, 359–386 (2015).
Carter, B. S., Beaty, T. H., Steinberg, G. D., Childs, B. & Walsh, P. C. Mendelian inheritance of familial prostate cancer. Proc. Natl Acad. Sci. USA 89, 3367–3371 (1992).
Mucci, L. A., Hjelmborg, J. B., Harris, J. R., Czene, K., Havelick, D. J., Scheike, T. et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 315, 68–76 (2016).
Schumacher, F. R., Al Olama, A. A., Berndt, S. I., Benlloch, S., Ahmed, M., Saunders, E. J. et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 50, 928–936 (2018).
Ewing, C. M., Ray, A. M., Lange, E. M., Zuhlke, K. A., Robbins, C. M., Tembe, W. D. et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 366, 141–149 (2012).
Struewing, J. P., Hartge, P., Wacholder, S., Baker, S. M., Berlin, M., McAdams, M. et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 336, 1401–1408 (1997).
Dong, X., Wang, L., Taniguchi, K., Wang, X., Cunningham, J. M., McDonnell, S. K. et al. Mutations in CHEK2 associated with prostate cancer risk. Am. J. Hum. Genet. 72, 270–280 (2003).
Cybulski, C., Górski, B., Debniak, T., Gliniewicz, B., Mierzejewski, M., Masojć, B. et al. NBS1 is a prostate cancer susceptibility gene. Cancer Res. 64, 1215–1219 (2004).
Na, R., Zheng, S. L., Han, M., Yu, H., Jiang, D., Shah, S. et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 71, 740–747 (2017).
Raymond, V. M., Mukherjee, B., Wang, F., Huang, S. C., Stoffel, E. M., Kastrinos, F. et al. Elevated risk of prostate cancer among men with Lynch syndrome. J. Clin. Oncol. 31, 1713–1718 (2013).
Zhang, F., Ma, J., Wu, J., Ye, L., Cai, H., Xia, B. et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr. Biol. 19, 524–529 (2009).
Reid, S., Schindler, D., Hanenberg, H., Barker, K., Hanks, S., Kalb, R. et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat. Genet. 39, 162–164 (2007).
Yang, X., Leslie, G., Doroszuk, A., Schneider, S., Allen, J., Decker, B. et al. Cancer risks associated with germline PALB2 pathogenic variants: an International Study of 524 Families. J. Clin. Oncol. 38, 674–685 (2020).
Cybulski, C., Kluźniak, W., Huzarski, T., Wokołorczyk, D., Kashyap, A., Rusak, B. et al. The spectrum of mutations predisposing to familial breast cancer in Poland. Int. J. Cancer 145, 3311–3320 (2019).
Cybulski, C., Kluźniak, W., Huzarski, T., Wokołorczyk, D., Kashyap, A., Jakubowska, A. et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol. 16, 638–644 (2015).
Lener, M. R., Scott, R. J., Kluźniak, W., Baszuk, P., Cybulski, C., Wiechowska-Kozłowska, A. et al. Do founder mutations characteristic of some cancer sites also predispose to pancreatic cancer? Int. J. Cancer 139, 601–606 (2016).
Łukomska, A., Menkiszak, J., Gronwald, J., Tomiczek-Szwiec, J., Szwiec, M., Jasiówka, M. et al. Recurrent mutations in BRCA1, BRCA2, RAD51C, PALB2 and CHEK2 in Polish patients with ovarian cancer. Cancers 13, 849 (2021).
Page, E. C., Bancroft, E. K., Brook, M. N., Assel, M., Battat, M. H. A., Thomas, S. et al. Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur. Urol. 76, 831–842 (2019).
Brandão, A., Paulo, P. & Teixeira, M. R. Hereditary predisposition to prostate cancer: from genetics to clinical implications. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21145036 (2020).
Cybulski, C., Carrot-Zhang, J., Kluźniak, W., Rivera, B., Kashyap, A., Wokołorczyk, D. et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat. Genet. 47, 643–646 (2015).
Cybulski, C., Wokołorczyk, D., Kluźniak, W., Jakubowska, A., Górski, B., Gronwald, J. et al. An inherited NBN mutation is associated with poor prognosis prostate cancer. Br. J. Cancer 108, 461–468 (2013).
Tischkowitz, M., Sabbaghian, N., Ray, A. M., Lange, E. M., Foulkes, W. D. & Cooney, K. A. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in hereditary prostate cancer. Prostate 68, 675–678 (2008).
Pakkanen, S., Wahlfors, T., Siltanen, S., Patrikoski, M., Matikainen, M., Tammela, T. et al. PALB2 variants in hereditary and unselected Finnish Prostate cancer cases. J. Negat. Results Biomed. 8, 12 (2009).
Matejcic, M., Patel, Y., Lilyquist, J., Hu, C., Lee, K. Y., Gnanaolivu, R. D. et al. Pathogenic variants in cancer predisposition genes and prostate cancer risk in men of African ancestry. JCO Precis. Oncol. 4, 32–43 (2020).
Leongamornlert, D. A., Saunders, E. J., Wakerell, S., Whitmore, I., Dadaev, T., Cieza-Borrella, C. et al. Germline DNA repair gene mutations in young-onset prostate cancer cases in the UK: evidence for a more extensive genetic panel. Eur. Urol. 76, 329–337 (2019).
Southey, M. C., Goldgar, D. E., Winqvist, R., Pylkäs, K., Couch, F., Tischkowitz, M. et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J. Med. Genet. 53, 800–811 (2016).
Wu, Y., Yu, H., Li, S., Wiley, K., Zheng, S. L., LaDuca, H. et al. Rare germline pathogenic mutations of DNA repair genes are most strongly associated with grade group 5 prostate cancer. Eur. Urol. Oncol. 3, 224–230 (2020).
Pritchard, C. C., Mateo, J., Walsh, M. F., De Sarkar, N., Abida, W., Beltran, H. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
Leongamornlert, D., Saunders, E., Dadaev, T., Tymrakiewicz, M., Goh, C., Jugurnauth-Little, S. et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer 110, 1663–1672 (2014).
Darst, B. F., Dadaev, T., Saunders, E., Sheng, X., Wan, P., Pooler, L. et al. Germline sequencing DNA repair genes in 5,545 men with aggressive and non-aggressive prostate cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa132 (2020).
Heikkinen, T., Kärkkäinen, H., Aaltonen, K., Milne, R. L., Heikkilä, P., Aittomäki, K. et al. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin. Cancer Res. 15, 3214–3222 (2009).
Deng, M., Chen, H. H., Zhu, X., Luo, M., Zhang, K., Xu, C. J. et al. Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int. J. Cancer 145, 1517–1528 (2019).
de Bono, J., Mateo, J., Fizazi, K., Saad, F., Shore, N., Sandhu, S. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Grellety, T., Peyraud, F., Sevenet, N., Tredan, O., Dohollou, N., Barouk-Simonet, E. et al. Dramatic response to PARP inhibition in a PALB2-mutated breast cancer: moving beyond BRCA. Ann. Oncol. 31, 822–823 (2020).
Acknowledgements
We thank Daria Zanoza and Ewa Putresza for their help with managing databases.
Members of the Polish Hereditary Prostate Cancer Consortium
Bartłomiej Masojć8, Adam Gołąb9, Bartłomiej Gliniewicz10, Andrzej Sikorski9, Marcin Słojewski9, Jerzy Świtała10, Tomasz Borkowski11, Andrzej Borkowski11, Andrzej Antczak12, Łukasz Wojnar12, Jacek Przybyła13, Marek Sosnowski13, Bartosz Małkiewicz14, Romuald Zdrojowy14, Paulina Sikorska-Radek15, Józef Matych15, Jacek Wilkosz16, Waldemar Różański16, Jacek Kiś17, Krzysztof Bar17, Piotr Bryniarski18, Andrzej Paradysz18, Konrad Jersak19, Jerzy Niemirowicz19, Piotr Słupski20, Piotr Jarzemski20, Michał Skrzypczyk21, Jakub Dobruch21, Michał Puszyński9, Michał Soczawa9, Mirosław Kordowski10, Marcin Życzkowski18, Andrzej Borówka21, Joanna Bagińska22, Kazimierz Krajka22, Małgorzata Stawicka23, Olga Haus24, Hanna Janiszewska24, Agnieszka Stembalska25, Maria Małgorzata Sąsiadek25.
Author information
Authors and Affiliations
Consortia
Contributions
D.W. designed the study, analysed the study data and drafted the manuscript. W.K., K.S., B.R., K.G. and S.M. selected and prepared DNA samples for genotyping and performed genotyping. T.H., J.G., T.D., M.S. and C.C. enrolled patients and controls for the study, and collected phenotypic data for the study. A.K. performed statistical analyses. W.K. and A.J. performed NGS sequencing. P.D. selected, prepared and provided tissue samples and performed LOH analysis. M.R.A. performed bioinformatics analysis of NGS sequencing data and assisted in drafting the manuscript. S.A.N. and J.L. assisted in coordination of the study and in drafting the manuscript. C.C. conceived, designed and coordinated the study and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Pomeranian Medical University in Szczecin (IRB No. KB-0012/97/17). Patient clinical data have been obtained in a manner conforming with IRB ethical guidelines. Informed consent was obtained from all individual participants included in the study.
Data availability
The main research data supporting the results of this study are included in Tables 1–3 and Figs. 1 and 2. Other data can be made available upon reasonable request from the corresponding author.
Competing interests
S.N. is an editorial board member for the British Journal of Cancer. All other authors declare no competing interests.
Funding information
This study was funded by National Science Centre, Poland; project number: 2015/19/B/NZ2/02439.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wokołorczyk, D., Kluźniak, W., Stempa, K. et al. PALB2 mutations and prostate cancer risk and survival. Br J Cancer 125, 569–575 (2021). https://doi.org/10.1038/s41416-021-01410-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01410-0
This article is cited by
-
Impact of germline mutations in cancer-predisposing genes on long-term survival in patients with epithelial ovarian cancer
British Journal of Cancer (2022)
-
An appraisal of genetic testing for prostate cancer susceptibility
npj Precision Oncology (2022)