Abstract
Objectives To evaluate the clinical-effectiveness of oral splints for patients with TMD or bruxism for the primary outcomes: pain (TMD) and tooth wear (bruxism).
Data sources Four databases including MEDLINE and EMBASE were searched from inception until 1 October 2018.
Data selection and extraction Randomised controlled trials comparing all types of splints versus no/minimal treatment for patients with TMD or bruxism were eligible. Standard Cochrane review methods were used. Standardised mean differences (SMD) were pooled for the primary outcome of pain, using random effects models in TMD patients.
Data synthesis Thirty-seven trials were included and the evidence identified was of very low certainty using GRADE assessments. When all subtypes of TMD were pooled into one global TMD group, there was no evidence that splints reduced pain: SMD (up to 3 months) -0.18 (95% CI -0.42 to 0.06); 13 trials, 1,076 participants. There was no evidence that any other outcomes improved when using splints. There was no evidence of adverse events associated with splints, but reporting was poor regarding this outcome. No trials measured tooth wear in patients with bruxism. There was a large variation in diagnostic criteria, splint types and outcome measures used and reported. Sensitivity analyses based on these factors did not indicate a reduction in pain.
Conclusions The very low-certainty evidence identified did not demonstrate that splints reduced pain in TMD as a group of conditions. There is insufficient evidence to determine whether splints reduce tooth wear in patients with bruxism.
Similar content being viewed by others
Key points
-
This systematic review comprehensively summarised the best available evidence from randomised controlled trials on the effects of oral splints for temporomandibular disorders (TMD) and bruxism.
-
There is no evidence to support the use of oral splints for either condition based on the results found.
-
For TMD patients, sensitivity analyses were conducted to explore the effects of differences in: 1) diagnostic criteria; 2) splint types; and 3) outcome measures used and reported. There were no differences in the results based on these factors.
Introduction
Temporomandibular disorders (TMD) are the second most common cause (after dental pain) of orofacial pain, characterised by pain in the temporomandibular joint area and in the facial muscles. Apart from pain, patients may experience other signs and symptoms, such as clicking of the joint and restricted mouth-opening. Around 5% to 12% of the population have TMD symptoms to some degree, varying by age group and gender.1 One of the most common ways in which dentists, particularly in primary care, manage symptomatic TMD is the provision of oral splints.2
Splints are also provided to help manage tooth wear caused by bruxism. The prevalence of bruxism ranges from 8% to 31% within the general population,3 and it is estimated globally that sleep bruxism affects 16%, and awake bruxism 24%, of the adult population.4
There is continuing debate about the exact mechanism of action of oral splints. Mechanisms include: muscle relaxation/habit-breaking for patients with increased parafunctional or muscle-tightening habits; protection of teeth and jaws, particularly where teeth clenching and grinding may lead to damage of teeth; normalising periodontal ligament proprioception, by utilising a splint to spread the forces placed on individual teeth; and repositioning of the jaws and condyles into centric relation.
This systematic review arose from an National Institute for Health Research Health Technology Assessment call addressing the research question: 'What is the clinical and cost-effectiveness of prefabricated oral splints and custom-made splints for the treatment of orofacial symptoms?' (in press). This research presents part of this review, looking at whether oral splints are effective in reducing orofacial symptoms (primarily pain) and when they are indicated to prevent tooth wear.
Methods
We undertook the review using Cochrane methods,5 which are described in greater detail elsewhere (in press).
Eligibility criteria
Randomised controlled trials were included (crossover studies were excluded as deemed inappropriate). We included children (over 11 years old) and adults who had either TMD or bruxism, in either primary or secondary care.
We included trials where any type of splint was compared with a non-splint group. This group also included watchful waiting or minimal treatment (advice/counselling, education or self-performed exercises).
The primary outcomes were pain and harms. For bruxism patients, tooth wear was also considered a primary outcome. Secondary outcomes included clicking of the temporomandibular joint, change in restricted mouth opening, frequency of headaches and reduced quality of life. Patient satisfaction and adherence to treatment were collected whenever possible. For bruxism, the index and frequency of bruxism activity was also to be recorded.
Follow-up periods for the outcome data were divided into short-term follow-up (0 to 3 months), medium-term (>3 to 6 months), or long-term (>6 to 12 months).
Search methods for identification of studies
An information specialist developed a search strategy (see online-only Supplementary Appendix 1) and conducted the literature searches on 1 October 2018. They were undertaken without restrictions on language or date of publication.
The following databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, and CINAHL EBSCO. Unpublished data were sought via searches of the US National Institutes of Health trials register (ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform. Conference proceedings were searched via Embase and the Web of Science. Abstracts of dissertations and theses were searched via the Proquest database. Additional grey literature was sourced through the American Academy of Dental Sleep Medicine (AADSM; http://www.aadsm.org/) website. The International Association of Dental Research (IADR) annual conference abstracts were searched via the IADR website.
Selection of studies and data extraction
Two review authors independently assessed studies retrieved by the searches for eligibility. Disagreements on study eligibility were resolved through discussion and consensus. If necessary, a third review author was consulted. Two review authors independently extracted the following data from the included trials: location/setting, type of provider, number of centres, recruitment period, trials registry ID, inclusion/exclusion criteria, demographic information, presenting condition and severity, type of splint, details of comparator, outcomes reported (including method and time of assessment), details of sample size calculations, funding sources and declarations/conflicts of interest.
Assessment of risk of bias in included studies
The assessment of risk of bias was done independently and in duplicate, using the Cochrane risk of bias tool.5 The following domains were assessed: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other bias. The overall risk of bias of individual studies was categorised as: low (plausible bias unlikely to seriously alter the results), if all domains were at low risk of bias; unclear (plausible bias that raises some doubt about the results), if one or more domains had an unclear risk of bias; or high, if one or more domains had a high risk of bias.
Statistical methods
For continuous outcomes, we used the means and standard deviations reported in the trials to express the estimate of effect as mean difference with 95% confidence interval (CI). If different scales were reported, we used standardised mean difference (SMD). For dichotomous outcomes, we expressed the estimate of effect as a risk ratio with 95% CI.
We attempted to contact the author(s) of all included studies, where feasible, in the event of missing data. Missing standard deviations were estimated.5
We assessed heterogeneity statistically by using a Chi² test, where a P value of less than 0.1 indicates statistically significant heterogeneity. We quantified heterogeneity by using the I² statistic.
We combined mean differences (or SMDs) for continuous data, and risk ratios for dichotomous data, using random effects models.
Sensitivity analysis
For TMD patients, we undertook a sensitivity analysis restricted to trials where the inclusion criteria were based on, or could be clearly mapped to, one of the following sets of diagnostic criteria: Research Diagnostic Criteria for Temporomandibular disorders (RDC/TMD) guidelines;6 TMD (DC/TMD) guidelines;7and American Association of Orofacial Pain (AAOP) guidelines.8 The outcome measures used and reported varied between studies; therefore, a sensitivity analysis was also carried out including only studies that measured pain at the time of assessment (current pain) on a 0 to 100 visual analogue or numerical rating scale. We also carried out sensitivity analysis based on splint types, restricting the analyses to studies using stabilisation splints.
Similarly, for bruxism patients, we planned to undertake a sensitivity analysis restricted to trials with a clear diagnosis of bruxism.9 The study should have used polysomnography (PSG) to diagnose the bruxism. There were insufficient trials to do this.
Presentation of main results
The certainty of the body of evidence was assessed following GRADE methods,10 considering the overall risk of bias of included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates and the risk of publication bias. We categorised the certainty of the body of evidence for each of the main outcomes for each comparison as high, moderate, low or very low.
Results
Characteristics of included studies
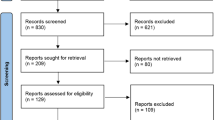
Thirsty-seven studies were included (Fig. 1); 34 on patients with TMD and two on patients with bruxism, with a further study on patients with both TMD and bruxism. All studies, with the exception of one, were conducted in universities or public hospitals/clinics.
For the studies evaluating the effectiveness of splints for people with TMD, the diagnostic criteria for TMD varied. However, the predominantly used criteria were the RDC,6 used in 17 studies.11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 The DC criteria7 were used in two studies,28,29 and an additional three studies used criteria that approximated to the RDC (either by citing the instrument and/or their description matched a similar process).30,31,32 No studies used the AAOP criteria.8
The remaining studies used criteria that we had not pre-specified in our protocol or that were undefined/unclear:
The two studies45,46 examining the effects of splints on bruxism used the Lobbezzo et al.9 criteria for likelihood of a bruxism diagnosis: 'possible' self-report of bruxism; 'probable' clinical evidence of bruxism with or without self-report; and 'definite' defined by polysomnography. We classified both studies as examining 'probable' bruxism.
The study that examined bruxism with co-morbid TMD used the Fonseca index for TMD and examined 'probable' bruxism.47
Thirty-five studies compared splints against no splints for TMD patients. Ten of these studies used a no treatment control group.14,23,30,32,33,34,35,37,41,43 Twenty had a co-intervention in each arm, with 13 having a 'minimal' co-intervention of usual treatment, counselling, information or exercise,11,12,13,15,16,17,18,19,21,22,25,26,36 while 7 had a 'non-minimal' co-intervention of 'acuhealth', manipulative and physical therapy, massage, Prozac, microcurrent electrical nerve stimulation, physical therapy with vapocoolant spray, arthrocentesis and sodium hyaluronate.27,28,29,39,40,44,47 The remaining six studies had minimal treatment controls: three were self-exercises,24,28,42 and three were information-based.20,27,31
One trial that has been referenced twice above27 had four arms with which we made two separate pairwise comparisons: 1) splint + co-intervention vs co-intervention alone; and 2) splint vs minimal treatment.
Nineteen studies used a stabilisation splint, 14 of which were in the upper jaw (Michigan-style splints),11,13,14,19,20,21,26,27,35,38,40,42,45,47 but not clearly reported in the other five.16,22,24,29,30
Seven studies compared more than one splint against no splint in this comparison, and were included twice in any meta-analysis as two separate pairwise comparisons.
For more details on the characteristics of the included studies, see online-only Supplementary Appendix 2.
Risk of bias
All 37 studies were assessed as being at high risk of bias overall due to a rating of high risk for at least one of the seven domains assessed (see Risk of bias summary in online-only Supplementary Appendix 3).
Ten studies were at low risk of selection bias because they adequately described methods used both to generate a truly random sequence and also to conceal the sequence from those involved in the randomisation process. The remaining studies were at unclear risk of selection bias as they had an unclear rating for either random sequence generation, allocation concealment, or both.
All studies, unavoidably, were at high risk of performance bias due to the comparison of splint against no splint. Thirty-five studies were at high risk of detection bias because patients knew their assigned group and also subjectively rated their own pain. The remaining two studies had a low risk of detection bias, the first being due to objective assessment of bruxism while the patients slept,46 and the second because no outcomes of this review were assessed and therefore this bias domain was irrelevant.47
Nine studies were at high risk of attrition bias due to high rates of attrition, large differences in attrition rates between groups, or both. The remaining six studies had an unclear risk of attrition bias due to poor reporting of numbers randomised or analysed.
Sixteen studies had problems with the way in which data were reported and were at high risk of reporting bias.
For other potential sources of bias, three studies were given a rating of high risk because outcomes were followed up at different times for the two groups. A further three studies were given an unclear rating for this domain because the reporting was poor and we were unable to properly assess them.
For more details on the risk of bias of the included studies, see online-only Supplementary Appendix 2.
Patients with TMD
There was consensus with clinicians and methodologists that 0 to 3 months was an appropriate time point to use for the primary analysis of the data. The primary pain outcome was any continuous scale that was sensible to combine (for example, Visual Analogue Scale (VAS), Numerical Rating Scale (NRS), Characteristic Pain Intensity (CPI)).
Thirteen trials of 16 pairwise comparisons, all at high risk of bias and with 1,076 patients, contributed to the results for the main comparison at three months (Table 1). There was considerable heterogeneity and the overall SMD was -0.18 (95% CI -0.42 to 0.06). Using a rule of thumb for SMD effect estimates,5 0.18 would be considered a small effect and, as this was not statistically significant, there is no evidence that oral splints reduce pain. Due to differences in splint type, the control group with no/minimal interventions and different types of TMD diagnoses between the individual studies, we were unable to investigate the heterogeneity any further. There were fewer studies and patients for the other time periods (>3 to 6 months: 2 trials, 160 patients and >6 to 12 months: 2 trials, three pairwise comparisons, 246 patients) and the effect sizes also failed to demonstrate that oral splints reduced pain.
There was no evidence of adverse events associated with splints, but reporting was poor regarding this outcome.
There was also no evidence that splints reduced TMD clicking or increased mouth opening, or improved quality of life, at any of the time points measured (online-only Supplementary Appendix 4). The certainty of the evidence for all these other outcomes was assessed as very low.
For TMD patients, we planned to undertake a sensitivity analysis restricted to trials where the inclusion criteria were based on, or could be clearly mapped to, one of the sets of diagnostic criteria mentioned in the Methods section. For the primary analysis of splints versus no/minimal intervention at 0 to 3 months (Table 1), there was no difference in the result when removing those trials that did not use the above diagnostic criteria: SMD -0.24 (95% CI -0.52 to 0.04; P = 0.09; I2 = 71%; 851 participants).
We carried out a sensitivity analysis restricting the meta-analysis in Table 1 to studies that measured pain at the time of assessment (current pain) measured on a 0 to 100 VAS or NRS. The results were consistent with the main SMD results: MD -4.48 (95% CI -11.59 to 2.64; P = 0.22; I2 = 94%; 874 participants).
We also carried out a sensitivity analysis restricting the meta-analysis in Table 1 to studies using stabilisation splints. Again, this did not change the result: SMD 0.04 (95% CI -0.13 to 0.22; P = 0.62; I2 = 27%; 750 participants). This removed much of the heterogeneity seen in the other analyses.
Patients with bruxism
Only one of the studies focusing on patients with bruxism provided usable outcome data at 0 to 3 months;45 however, no studies looked at the primary outcome tooth wear. The aforementioned study on 78 patients looked at the other primary outcome pain on a 0 to 10 scale and indicated that splints reduced pain MD -2.01 (95% CI -2.62, -1.40).
Discussion
Summary of main results
Despite the inclusion of 35 studies comparing oral splints to no splints or a minimal intervention in patients with TMD, the body of evidence was assessed as being at very low certainty (see Summary of findings table in online-only Supplementary Appendix 5). There was no evidence that oral splints improved the following outcomes: pain; clicking of the temporomandibular joint; restricted mouth opening; or quality of life.
For patients with bruxism, there was insufficient evidence to conclude whether the provision of oral splints reduced tooth wear, as no studies reported this. Although a small number of studies reported pain and other outcomes, there was also insufficient evidence to conclude whether or not oral splints were beneficial. We were unable to undertake any sensitivity analyses due to the lack of outcome data.
For the TMD patients, we undertook three separate sensitivity analyses restricted to trials where: a) the inclusion criteria were based on, or could be clearly mapped to, specific pre-determined sets of diagnostic criteria; b) only stabilisation splints were used; and c) current pain was measured on a 0 to 100 visual analogue scale or numerical rating scale. There were no differences in the results based on these factors.
For both patients with TMD and bruxism, due to differences in the diagnoses of the included trial participants and differences in the types of splints and control groups used, the applicability of the evidence is questionable and certainly incomplete for patients with bruxism.
Pain was reported in numerous different ways, at different times, and this reduced the number of studies that could be combined in a meta-analysis to produce a pooled estimate. The use of an agreed measure for pain and how and when this is measured would enable the pain data from all studies to contribute to a single pooled estimate. It is also important to consider what would be a clinically important reduction in pain. It is suggested that a reduction of around 20% represents a minimally important decrease, 30% a moderately important decrease and 50% a substantial decrease.48
Numerous studies reported on some of our outcomes but did not report the data in a suitable format for inclusion in our meta-analyses (including missing standard deviations). This can mean that meta-analyses are biased due to missing information. This highlights the need for standardisation in both 'what to measure' and 'how to measure it' in clinical trials within this area of research; otherwise, there will continue to be research waste, with data that we are unable to pool in data syntheses. Initiatives such as IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials), COMET (Core Outcome Measures in Effectiveness Trials) and COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) could help with these issues.
Certainty of the evidence
The certainty of the evidence for comparing splints with no splints/minimal interventions in patients with all sub-types of TMD was downgraded to 'very low' due to the studies being of high risk of bias, heterogeneity and lack of precision in the estimates (see online-only Supplementary Appendix 5). Most studies were assessed as being at high risk of bias due to the inability of researchers to blind patients to wearing a splint or not. As the primary outcome for the TMD patients was pain assessed by the patients themselves, this meant that outcome measurement was also assessed as being at high risk of bias. This risk of bias does not necessarily reflect how well the studies have been conducted as it is not possible to design trials to overcome this problem. However, the bias should still be acknowledged when considering our overall certainty of the evidence. If blinding was to be disregarded, our certainty of the evidence would still remain low.
There were no studies looking at tooth wear, and very few studies and lack of useable other data for the patients with bruxism; therefore we were unable to determine whether splints were effective in these patients.
The risk of bias for all the studies was high. Although patient blinding is not possible when comparing oral splints with no splints or a minimal intervention, there were also problems with selective reporting bias and incomplete outcome data.
Alternative treatment options for TMD
Other recent research into treatment options for the management of TMD has included orthognathic surgery,49 TMJ lavage,50 physiotherapy,51,52 low-level laser therapy,53,54 exercise therapy,55 pharmacological treatment, 56 and acupuncture. 57 However, results are mixed and generally unconvincing.
Conclusions
Implications for healthcare
From this systematic review, there is no clear evidence to support the provision of splints for the various sub-types of TMD or bruxism. However, the body of evidence that this conclusion is based on is of very low certainty. The studies included in this review differed in three important factors: 1) diagnoses, 2) splint type, and 3) outcome measurement/reporting. This made it difficult to draw clear and definitive conclusions.
Recommendations for future research
Further well-conducted randomised controlled trials are urgently needed to determine whether the use of splints is clinically effective, generates meaningful patient benefit and whether splints offer an efficient use of resources in both Bruxism and TMD. Multiple trials will be required to concentrate on specific sub-types of TMD in order to facilitate future, more focused meta-analyses. The need for further trials is perhaps more pronounced in bruxism patients, as there were no trials measuring tooth wear.
References
National Institute of Dental and Craniofacial Research (NIDCR). Prevalence of TMJD and its Signs and Symptoms. 2014. Available at https://www.nidcr.nih.gov/datastatistics/finddatabytopic/facialpain/prevalencetmjd.htm (accessed September 2019).
Aggarwal V R, Joughin A, Zakrzewska J, Appelbe P, Tickle M. Dentists' preferences for diagnosis, management and referral of chronic oro-facial pain: Results from a national survey. Health Educ J 2012; 71: 662-669.
Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain 2013; 27: 99-110.
Lobbezoo F, Ahlberg J, Manfredini D, Winocur E. Are bruxism and the bite causally related? J Oral Rehabil 2012; 39: 489-501.
Higgins J T, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from https://training.cochrane.org/handbook/archive/v5.1/ (accessed September 2019).
Dworkin S F, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992; 6: 301-355.
Schiffman E, Ohrbach R, Truelove E et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014; 28: 6-27.
American Academy of Orofacial Pain. De Leeuw R, Klasser G D (eds) Orofacial pain: guidelines for assessment, diagnosis, and management. 5th edition. Hanover Park, IL: Quintessence, 2013.
Lobbezoo F, Ahlberg J, Glaros A G et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013; 40: 2-4.
Atkins D, Best D, Briss P A et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490.
Conti P C, de Alencar E N, da Mota Correa A S, Lauris J R, Porporatti A L, Costa Y M. Behavioural changes and occlusal splints are effective in the management of masticatory myofascial pain: a short-term evaluation. J Oral Rehabil 2012; 39: 754-760.
Conti P C, Correa A S, Lauris J R, Stuginski-Barbosa J. Management of painful temporomandibular joint clicking with different intraoral devices and counseling: a controlled study. J Appl Oral Sci 2015; 23: 529-535.
Costa Y M, Porporatti A L, Stuginski-Barbosa J, Bonjardim L R, Conti P C. Additional effect of occlusal splints on the improvement of psychological aspects in temporomandibular disorder subjects: A randomized controlled trial. Arch Oral Biol 2015; 60: 738-744.
de Felicio C M, de Oliveira M M, da Silva M A. Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio 2010; 28: 249-259.
DeVocht J W, Goertz C M, Hondras M A et al. A pilot study of a chiropractic intervention for management of chronic myofascial temporomandibular disorder. J Am Dent Assoc 2013; 144: 1154-1163.
Ficnar T, Middelberg C, Rademacher B, Hessling S, Koch R, Figgener L. Evaluation of the effectiveness of a semi-finished occlusal appliancea randomized, controlled clinical trial. Head Face Med 2013; DOI: 10.1186/1746-160X-9-5.
Giannakopoulos N N, Katsikogianni E N, Hellmann D et al. Comparison of three different options for immediate treatment of painful temporomandibular disorders: a randomized, controlled pilot trial. Acta Odontol Scand 2016; 74: 480-486.
Hasanoglu Erbasar G N, Alpaslan C, Eroglu Inan G. Can an NTI-tss device be effective as a first-line therapy in patients with TMD myofascial pain? J Oral Rehabil 2017; 44: 589-593.
Katyayan P A, Katyayan M K, Shah R J, Patel G. Efficacy of appliance therapy on temporomandibular disorder related facial pain and mandibular mobility: a randomized controlled study. J Indian Prosthodont Soc 2014; 14: 251-261.
Michelotti A, Iodice G, Vollaro S, Steenks M H, Farella M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J Am Dent Assoc 2012; 143: 47-53.
Nagata K, Maruyama H, Mizuhashi R et al. Efficacy of stabilisation splint therapy combined with non-splint multimodal therapy for treating RDC/TMD axis I patients: a randomised controlled trial. J Oral Rehabil 2015; 42: 890-899.
Niemela K, Korpela M, Raustia A, Ylostalo P, Sipila K. Efficacy of stabilisation splint treatment on temporomandibular disorders. J Oral Rehabil 2012; 39: 799-804.
Nitecka-Buchta A, Marek B, Baron S. CGRP plasma level changes in patients with temporomandibular disorders treated with occlusal splints - a randomised clinical trial. Endokrynol Pol 2014; 65: 217-223.
Tavera A T, Montoya M C, Calderon E F, Gorodezky G, Wixtrom R N. Approaching temporomandibular disorders from a new direction: a randomized controlled clinical trial of the TMDes ear system. Cranio 2012; 30: 172-182.
Truelove E, Huggins K H, Mancl L, Dworkin S F. The efficacy of traditional, low-cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. J Am Dent Assoc 2006; 137: 1099-1107.
Wahlund K, List T, Larsson B. Treatment of temporomandibular disorders among adolescents: a comparison between occlusal appliance, relaxation training, and brief information. Acta Odontol Scand 2003; 61: 203-211.
Yu C H, Qian H X. Evaluation of short term efficacy of the stabilized splint and the combination of manipulative and physical therapies for temporomandibular joint disc displacement without reduction. [Chinese]. J Shanghai Jiaotong University (Medical Science) 2016; 36: 850-855.
Sharma A R. Short term efficacy of appliance and physical therapy for the treatment of bilateral masseter myalgia: A double blinded randomized clinical trial. Ann Arbor: State University of New York at Buffalo, 2016. Dissertation.
Tatli U, Benlidayi M E, Ekren O, Salimov F. Comparison of the effectiveness of three different treatment methods for temporomandibular joint disc displacement without reduction. Int J Oral Maxillofac Surg 2017; 46: 603-609.
Conti P C, Miranda J E, Conti A C, Pegoraro L F, Araujo Cdos R. Partial time use of anterior repositioning splints in the management of TMJ pain and dysfunction: a one-year controlled study. J Appl Oral Sci 2005; 13: 345-350.
de Felicio C M, Mazzetto M O, de Silva M A, Bataglion C, Hotta T H. A preliminary protocol for multi-professional centres for the determination of signs and symptoms of temporomandibular disorders. Cranio 2006; 24: 258-264.
Wright E, Anderson G, Schulte J. A randomized clinical trial of intraoral soft splints and palliative treatment for masticatory muscle pain. J Orofac Pain 1995; 9: 192-199.
Daif E T. Correlation of splint therapy outcome with the electromyography of masticatory muscles in temporomandibular disorder with myofascial pain. Acta Odontol Scand 2012; 70: 72-77.
Johansson A, Wenneberg B, Wagersten C, Haraldson T. Acupuncture in treatment of facial muscular pain. Acta Odontol Scand 1991; 49: 153-158.
List T, Helkimo M, Andersson S, Carlsson G E. Acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. Part I. A comparative study. Swed Dent J 1992; 16: 125-141.
Lundh H, Westesson P L, Jisander S, Eriksson L. Disk-repositioning onlays in the treatment of temporomandibular joint disk displacement: comparison with a flat occlusal splint and with no treatment. Oral Surg Oral Med Oral Pathol 1988; 66: 155-162.
Lundh H, Westesson P L, Eriksson L, Brooks S L. Temporomandibular joint disk displacement without reduction. Treatment with flat occlusal splint versus no treatment. Oral Surg Oral Med Oral Pathol 1992; 73: 655-658.
Haketa T, Kino K, Sugisaki M, Takaoka M, Ohta T. Randomized clinical trial of treatment for TMJ disc displacement. J Dent Res 2010; 89: 1259-1263.
Elsharkawy T M, Ali N M. Evaluation of acupuncture and occlusal splint therapy in the treatment of temporomandibular joint disorders. Egypt Dent J 1995; 41: 1227-1232.
Leeson R M A. A comparison of medical and physical therapies in the management of facial arthromyalgia (temporomandibular joint dysfunction). Ann Arbor: University of London, 2007. Doctoral Thesis.
Lundh H, Westesson P L, Kopp S, Tillstrom B. Anterior repositioning splint in the treatment of temporomandibular joints with reciprocal clicking: comparison with a flat occlusal splint and an untreated control group. Oral Surg Oral Med Oral Pathol 1985; 60: 131-136.
Magnusson T, Syren M. Therapeutic jaw exercises and interocclusal appliance therapy. A comparison between two common treatments of temporomandibular disorders. Swed Dent J 1999; 23: 27-37.
Rampello A, Saccucci M, Falisi G, Panti F, Polimeni A, Di Paolo C. A new aid in temporomandibular joint disorders' therapy: the universal neuromuscular immediate relaxing appliance. J Biol Regul Homeost Agents 2013; 27: 1011-1019.
Zuim P R, Garcia A R, Turcio K H, Hamata M M. Evaluation of microcurrent electrical nerve stimulation (MENS) effectiveness on muscle pain in temporomandibular disorders patients. J Appl Oral Sci 2006; 14: 61-66.
Gomes C A, El-Hage Y, Amaral A P et al. Effects of Massage Therapy and Occlusal Splint Usage on Quality of Life and Pain in Individuals with Sleep Bruxism: A Randomized Controlled Trial. J Jpn Phys Ther Assoc 2015; 18: 1-6.
Pierce C J, Gale E N. A comparison of different treatments for nocturnal bruxism. J Dental Res 1988; 67: 597-601.
Gomes C A, El Hage Y, Amaral A P, Politti F, Biasotto-Gonzalez D A. Effects of massage therapy and occlusal splint therapy on electromyographic activity and the intensity of signs and symptoms in individuals with temporomandibular disorder and sleep bruxism: a randomized clinical trial. Chiropr Man Therap 2014; 22: 43.
Dworkin R H, Turk D C, Wyrwich K W et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105-121.
Al-Moraissi E A, Wolford L M, Perez D, Laskin D M, Ellis E 3rd. Does orthognathic surgery cause or cure temporomandibular disorders? A systematic review and meta-analysis. J Oral Maxillofac Surg 2017; 75: 1835-1847.
Bouchard C, Goulet J P, El-Ouazzani M, Turgeon A F. Temporomandibular lavage versus nonsurgical treatments for temporomandibular disorders: a systematic review and meta-analysis. J Oral Maxillofac Surg 2017; 75: 1352-1362.
Calixtre L B, Moreira R F, Franchini G H, Alburquerque-Sendin F, Oliveira A B. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trials. J Oral Rehabil 2015; 42: 847-861.
Paço M, Peleteiro B, Duarte J, Pinho T. The effectiveness of physiotherapy in the management of temporomandibular disorders: a systematic review and meta-analysis. J Oral Facial Pain Headache 2016; 30: 210-220.
Chang W D, Lee C L, Lin H Y, Hsu Y C, Wang C J, Lai P T. A meta-analysis of clinical effects of low-level laser therapy on temporomandibular joint pain. J Phys Ther Sci 2014; 26: 1297-1300.
Munguia F M, Jang J, Salem M, Clark G T, Enciso R. Efficacy of low-level laser therapy in the treatment of temporomandibular myofascial pain: a systematic review and meta-analysis. J Oral Facial Pain Headache 2018; 32: 287-297.
Dickerson S M, Weaver J M, Boyson A N et al. The effectiveness of exercise therapy for temporomandibular dysfunction: a systematic review and meta-analysis. Clin Rehabil 2017; 31: 1039-1048.
Häggman-Henrikson B, Alstergren P, Davidson T et al. Pharmacological treatment of oro-facial pain - health technology assessment including systematic review with network meta-analysis. J Oral Rehabil 2017; 44: 800-826.
Jung A, Shin B C, Lee M S, Sim H, Ernst E. Acupuncture for treating temporomandibular joint disorders: a systematic review and meta-analysis of randomized, sham-controlled trials. J Dent 2011; 39: 341-350.
Acknowledgments
Funded by: This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (Project number: 16/146/06). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health
The Health Services Research Unit and the Health Economics Research Unit are funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme [Project number: 16/146/06]. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of Interest:
There were no known conflicts of interest for any of the authors.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riley, P., Glenny, AM., Worthington, H. et al. Oral splints for temporomandibular disorder or bruxism: a systematic review. Br Dent J 228, 191–197 (2020). https://doi.org/10.1038/s41415-020-1250-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-020-1250-2
This article is cited by
-
Effects of different interventions on bruxism: an overview of systematic reviews
Sleep and Breathing (2024)
-
Recording the maxillomandibular relationship with the Aqualizer system prior to occlusal splint therapy for treating temporomandibular disorders: a randomized controlled trial
Scientific Reports (2023)
-
Effects of storage and toothbrush simulation on Martens hardness of CAD/CAM, hand-cast, thermoforming, and 3D-printed splint materials
Clinical Oral Investigations (2023)
-
Einfluss von aktiven Übungsprogrammen und Aufbissschienen auf die kraniomandibuläre Dysfunktion
Manuelle Medizin (2023)
-
Mechanical and chemical characterization of contemporary occlusal splint materials fabricated with different methods: a systematic review
Clinical Oral Investigations (2023)