Abstract
Low back pain (LBP) is the world’s leading cause of disability and is increasing in prevalence more rapidly than any other pain condition. Intervertebral disc (IVD) degeneration and facet joint osteoarthritis (FJOA) are two common causes of LBP, and both occur more frequently in elderly women than in other populations. Moreover, osteoarthritis (OA) and OA pain, regardless of the joint, are experienced by up to twice as many women as men, and this difference is amplified during menopause. Changes in estrogen may be an important contributor to these pain states. Receptors for estrogen have been found within IVD tissue and nearby joints, highlighting the potential roles of estrogen within and surrounding the IVDs and joints. In addition, estrogen supplementation has been shown to be effective at ameliorating IVD degeneration and OA progression, indicating its potential use as a therapeutic agent for people with LBP and OA pain. This review comprehensively examines the relationship between estrogen and these pain conditions by summarizing recent preclinical and clinical findings. The potential molecular mechanisms by which estrogen may relieve LBP associated with IVD degeneration and FJOA and OA pain are discussed.
Similar content being viewed by others
Introduction
Low back pain (LBP) and osteoarthritis (OA) are two of the most common, disabling, and costly health conditions.1 LBP affects up to 80% of people at some point in their lives, with the majority of episodes occurring at the lumbar or waist region of the spine, which bears the brunt of the weight of the upper body and explains why LBP is so common in humans compared to quadruped animals.2,3 Many studies highlight intervertebral disc (IVD) degeneration and facet joint osteoarthritis (FJOA) as two major contributors to LBP.4,5,6 OA, on the other hand, is a progressive degenerative disease that tends to worsen over time and, as a result, frequently leads to chronic pain. OA pain can affect various joints in the body, including the hips, knees, hands, and spine. Both of these pain conditions place enormous health and economic burdens on patients, their families, and society.7
LBP prevalence and incidence rates are higher in women than men.8,9 Moreover, middle-aged women after menopause are more likely to suffer from LBP than their younger counterparts. Similarly, women are more likely to suffer from OA than men, with the prevalence increasing after menopause10. Hormonal changes that occur in older women as estrogen production slows may have a role.11 In premenopausal women, estradiol levels are typically between 30 and 300 pg·mL−1, whereas in postmenopausal women, levels typically fall to between 0 and 40 pg·mL−1.12 Estrogen deficiency, which occurs in more than 50% of perimenopausal (just prior to menopause) women, is closely associated with decreased bone mineral density and increased musculoskeletal pain.13 Women with severe menopausal symptoms are more likely to suffer from chronic back pain and OA.10,14 Moreover, estrogen receptors have been identified within the IVDs and different joints.
These links between estrogen and musculoskeletal pain contribute to the growing argument that estrogen is much more than just a sex hormone. In this review, we provide an overview of the relationship between estrogen and these pain conditions by summarizing current preclinical and clinical findings. The potential molecular mechanisms by which estrogen may relieve LBP associated with IVD degeneration and FJOA and OA pain are discussed. New avenues of research are presented.
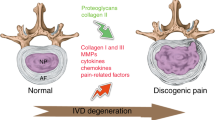
Intervertebral disc (IVD) degeneration
Like all musculoskeletal pain, LBP involves contributions from various biopsychosocial and lifestyle factors, especially as the pain continues to persist.15,16 One of the primary triggers, however, is IVD degeneration.6 IVD degeneration refers to a deterioration of the discs between vertebral bodies (hence the term “intervertebral”) of the spine, which can lead to discomfort, reduced range of motion, and various other symptoms associated with LBP.17,18 There are 23 IVDs in the human spine that lie between adjacent vertebrae, providing both the required support and elasticity for the spine.19 The IVD consists of three main components: the nucleus pulposus, the annulus fibrosus, and the cartilage endplate.20 IVDs function as vital fluid pumps, pushing water into the medullary canaliculi when under pressure and regaining water through the hydrophilic activity of proteoglycans when pressure is released.21 The viscoelasticity of IVDs decreases with age due to age-related reductions in the hydrophilic proteoglycan content and quality.22 IVD degeneration is characterized by reduced synthesis of proteoglycans, decreased water content, abnormal extracellular matrix (ECM) production, and improper collagen types.23 As the discs continue to lose elasticity and water content, they also lose their ability to resist compression and torque and maintain structural integrity. Compounding the problem is a cascade of consequential effects, including altered spinal biomechanics, neovascularization, and neoinnervation. These degenerative changes can alter the rest of the spinal column and adversely affect other spinal structures, such as muscles and ligaments, leading to the degeneration of adjacent tissues, nerve impingement (and radicular pain) and spinal stenosis, among other contributors to pain.24 These effects are amplified in older adults, as cells within IVDs and nearby tissues are more exposed to conditions (such as oxidative stress and damaged matrix products) that enhance cell death and senescence.25,26,27 Eventually, a pathologic state may be reached, and chronic pain occurs.23
Facet joint osteoarthritis (FJOA)
FJOA is a consequence of spinal degeneration and is considered another primary cause of LBP. FJOA is a whole-joint failure disease that results from injury to one or more components of the spinal motion segment.28,29,30,31 In humans, facet joints are a set of synovial joints located between the articular processes of two adjacent spinal levels. The facet joints and IVD, also known as the ‘three-joint complex’, constitute an important component of spinal motion segments.32 The ‘three-joint complex’ is crucial for enabling spinal motions, including rotation, flexion and extension.33
Osteoarthritis (OA)
OA is a degenerative joint disease, a common musculoskeletal disorder in elderly people, that is characterized by the deterioration of cartilage, along with alterations in the subchondral bone and the development of osteophytes, resulting in pain and physical disability.34,35,36 OA manifests itself in a variety of ways, including joint pain, stiffness, and restricted movement.37 Despite the slow progression of this disease, it can eventually cause joint failure and disability.10 Women between the ages of 50 and 60 years are 3.5 times more susceptible to developing hand OA than men in the same age group.38 Moreover, women have a 40% and 10% higher chance of developing knee OA and hip OA than men, respectively.39,40 In addition, initial studies suggest that women may experience more severe OA pain than men,41 but further research is needed.
Estrogen
Estrogen refers to a group of steroid hormones secreted from the ovaries that play important roles in numerous tissues throughout the human body.42 Estrogen has significant regulatory effects not only on the development, differentiation, and function of the female reproductive system but also on the metabolism of the musculoskeletal system.43,44,45 To date, four types of estrogens have been identified in humans, namely, estrone (E1), estradiol (E2, or 17β-E2), estriol (E3) and estetrol (E4).46,47 E1, E2 and E3 are the most common estrogens, with E2 being the most potent and E4 only being detectable during pregnancy, as it is produced by the fetal liver.47 Estrogen plays a critical role in bone health in both sexes. Estrogen promotes the activity of osteoblasts, which are cells that synthesize and secrete bone matrix and participate in the mineralization of bone.48 This function suggests that estrogen may help slow the breakdown of bones and encourage bone growth48 and that reductions in estrogen over time could compromise bone health.49
The lipophilic nature of estrogen means that it can penetrate cell membranes and bind to estrogen receptors (ERs) with high efficiency. These receptors include ER-alpha (ERα), ER-beta (ERβ) and the membrane-bound G-protein-coupled and seven-transmembrane receptor 30 (GPR30, also named G-protein coupled ER1).50 To date, research has identified the presence of ERs in various joint tissues,51 including ERα and ERβ in human IVD cells. The ERβ gene is expressed in the annulus fibrosis of IVDs, and both ERα and ERβ are present in the nucleus pulposus and cartilage endplate.52 Downregulation of ERα and ERβ levels has been observed with IVD degeneration.52,53 GPR30 is expressed in the nucleus pulposus of IVDs and plays an important role in E2-mediated nucleus pulposus cell proliferation and survival.54 However, clarification of which ER subtypes are most important in IVD degeneration requires further investigation.
Chondrocyte prostaglandin synthesis can be increased by estrogen treatment, while proteoglycan synthesis can be reduced.55 ERs have been identified in many joint components, including synovium, bone, cartilage, and ligaments,56,57 suggesting that estrogen has important roles in regulating and maintaining these tissues. The influence of estrogen on cartilage metabolism has been widely studied.58 For example, glycosaminoglycans, as complex carbohydrates, are widely and copiously expressed on the surface of cells and within the extracellular matrix. Glycosaminoglycan synthesis is important for normal joint and cartilage function. It has been shown that glycosaminoglycan synthesis is enhanced by treating cultured rabbit chondrocytes with E2.59 Furthermore, cyclooxygenase-2 expression was suppressed with E2 treatment in cultured bovine articular chondrocytes, which could protect cells from oxidative damage.60 However, findings do not always support the positive impacts of estrogen. Studies investigating ovariectomized (OVX) rats showed that knee cartilage damage could be prevented with estrogen treatment due to effects on limiting cartilage turnover,61,62,63 whereas the cartilage structure in estrogen-deficient rabbits continued to degrade even after intraarticular injections of estrogen.64,65 Furthermore, higher ER expression in facet joint cartilage was thought to aggravate the severity of FJOA in postmenopausal women.66 These findings highlight the complex roles of estrogen in regulating the metabolic homeostasis of the IVD and facet joints and that further studies are required to disentangle the mechanisms for each estrogen and receptor subtype.
The role of estrogen in LBP and OA pain
Estrogen deficiency and LBP
Estrogen deficiency may facilitate IVD degeneration. In 2004, it was shown that OVX rats had lower bone mineral density and bone volume indices but a higher bone turnover rate in the lumbar spine than those of sham-operated controls.67 The OVX group also had higher IVD degeneration histological scores, indicative of IVD degeneration.67 In another study comparing OVX versus non-OVX rats, IVD degeneration induced by facetectomy appeared to be exacerbated by low estrogen levels, as the affected vertebral body (C5) was substantially weaker (confirmed by imaging) in OVX rats.68 In addition, IVD shrinkage, oxidative stress, and autophagy were found in rat nucleus pulposus tissue after OVX surgery.69 Moreover, nucleus pulposus cells in non-OVX rats remain as notochord cells, whereas in OVX rats, they differentiate into chondrocyte-like cells.70 Other OVX studies have also shown reductions in endplate porosity, downregulation of aggrecan, type II collagen alpha 1 (COL2α1) and Wnt/β-catenin pathways, and changed biomechanical properties of the IVD.70 In addition, the progression of IVD degeneration was accelerated in OVX rats through changes including osteochondral remodeling of the cartilage endplate and vertebral osteoporosis.71 More importantly, the calcification level of the cartilage endplate was higher in the OVX plus vehicle group than that in the sham group and OVX plus E2 group, suggesting that decreased estrogen levels aggravate the degeneration of the cartilage endplate.72 It is worth noting that ERα and ERβ expression was found to decrease as IVD degeneration worsened in both males and females and that ERα and ERβ expression in the nucleus pulposus of males was significantly higher than that of females.52 To date, however, no in vivo animal studies/human studies have investigated the impact of estrogen replacement therapy on IVD degeneration in males. This may be due to the fact that relative to females, males have very low levels of estrogen and high levels of androgens, the latter of which also has critical roles in IVD maintenance.73,74 Further work is needed to reveal the full impact of estrogen on IVD metabolism in both males and females.
In addition to IVD degeneration, estrogen deficiency may also promote FJOA. Researchers comparing morphologic changes in cartilage and subchondral bone of the lumbar facet joint between OVX versus non-OVX mouse models showed that OVX mice had75 (1) cartilage with decreased surface area, thickness, and volume; (2) surface subchondral bone damage; and (3) increased osteoclasts within their subchondral bone, which was confirmed by another study.76 Furthermore, lumbar facet joint arthritis was accelerated under estrogen-deficient conditions in mice, as evidenced by the severe cartilage degradation, decreased subchondral bone mass of the lumbar facet joint, increased cavities on the subchondral bone interface, and increased angiogenesis and nerve ending growth in the degenerated lumbar facet joints.77
Clinical evidence supports these OVX animal findings. In a clinical study, 15 OVX patients and 60 non-OVX patients with LBP were compared regarding the severity of IVD degeneration using a modified Pfirrmann grading system, a scoring tool used for IVD degeneration evaluation.78 Non-OVX patients had lower scores at all IVD levels than OVX patients. Unilateral OVX patients had lower scores at levels L3/4 and L5/S1 relative to those of bilateral OVX patients. These data suggest that OVX may have, at least in part, contributed to the progression of IVD degeneration. A cross-sectional study comparing estrogen levels with respect to back pain and lumbar OA among 196 postmenopausal women showed that estrogen deficiency was correlated with symptomatic lumbar OA.79
Estrogen deficiency and OA
A number of studies support the role of estrogen deficiency in OA more broadly, including in the temporomandibular joint and knee joint.80 In OVX versus non-OVX animal studies, OVX animals had more extensive cartilage damage,62 erosion and subchondral bone changes,81 and increased surface erosion of the articular cartilage.61,82 Comparable alterations in cartilage structure have also been reported in other animal types, including rabbits,83 sheep,84, and guinea pigs.85 In 2008, a systematic review of OVX in animal OA models found that 11 of 16 studies reported significant cartilage damage in response to OVX.86 In a recent study, OVX mice developed severe posttraumatic osteoarthritis lesions and more extensive cartilage damage in the knee joint 8 weeks after destabilization of the medial meniscus surgery compared to non-OVX mice.87 These changes were supported by histological findings, including reduced safranin O staining (macroscopic observation and Mankin score), areas of defective uncalcified cartilage, and substantial decreases in both the area and thickness of the articular cartilage.87 In another study, the mRNA levels of early markers of osteogenic differentiation (e.g., type I collagen and Runx2) were shown to be elevated in OVX rats.81
Several human studies support the correlation between estrogen levels and OA.51 A meta-analysis found that women tended to experience more severe knee OA than men and that this was substantially amplified in women after menopause, a period characterized by marked and progressive declines in estrogen.88 Together with animal findings, these data highlight the potential pathological role of low estrogen levels in accelerating OA and OA pain.
Estrogen supplementation alleviates IVD degeneration and FJOA
As shown in Table 1, the potential for estrogen to reduce IVD degeneration and FJOA progression to relieve LBP of different etiologies has been demonstrated in many animal model studies.
The reduced density and connection of the cancellous bone of the C5 vertebral body in OVX rats with IVD degeneration were reversed after estrogen supplementation in rats with IVD degeneration.68 This finding may be explained by estrogen’s capacity to reduce oxidative stress and autophagy in the nucleus pulposus tissues, which is in part caused by estrogen deficiency.69 Thus, estrogen supplementation may be a possible therapeutic option for females with IVD degeneration. In another study, IVD shrinkage in a rat model induced by OVX was prevented by E2 treatment.89 It was found that the abnormal chondrocyte-like nucleus pulposus cells; reduced endplate porosity; expression of aggrecan, COL2α1, and Wnt/β-catenin pathway members; and altered biomechanical properties of IVDs caused by OVX surgery all improved after estrogen supplementation.70 A recent study showed that serum levels of E2 and IVD height in OVX rats increased to a similar level in the sham group after subcutaneous administration of E2 (10 μg·kg−1 per day).90 The reduced nucleus pulposus cells and ECM (including aggrecan and collagen) and ossified endplates were also reversed by E2 treatment.90 In another study, E2 (25 μg·kg−1) treatment reversed IVD cell apoptosis in OVX rats, and this E2 effect was abolished by treatment with ICI182780, an antagonist of the estrogen receptor.89 Moreover, targeted estrogen treatment has anti-inflammatory and antihyperalgesic effects on the nucleus pulposus tissue of IVDs in OVX mice with IVD degeneration.91
Estrogen replacement therapy is thought to mitigate and even prevent lumbar facet joint arthritis.77 Following E2 supplementation in OVX rats, arthritic pathological processes at the lumbar facet joint were suppressed, and cartilage degradation was inhibited. This result suggests that estrogen has an important role in maintaining the structural integrity of joints and that estrogen supplementation could protect against degeneration and even, in part, restore the integrity of the lumbar facet joint.77
The potential protective effects of estrogen on IVD degeneration in people with LBP are supported by data from clinical trials (Table 2). One study showed that menopausal women had substantially lower IVD height than premenopausal women and menopausal women receiving estrogen replacement therapy.92 In another study, postmenopausal women who were receiving E2 progesterone therapy, compared to those who were not, had a lower rate of lateral rotatory olisthesis, which is a potential trigger for degenerative scoliosis and LBP.93,94 Baron et al. showed that postmenopausal women presenting with osteoporotic vertebral fractures who were receiving estrogen-progestin treatment had significantly higher IVD heights relative to those who were not receiving treatment.95 Another cross-sectional study conducted in Korea examined the relationship between spinal OA prevalence and the use of hormone replacement therapy.96 Clearly, postmenopausal women undergoing hormone replacement therapy treatment had a lower prevalence of spinal OA. Moreover, women who had received treatment for more than 1 year were at an even lower risk than those who had received treatment for less time.96 Unfortunately, the authors did not provide exact details about the hormone therapies used (e.g., type or dose).96 These results suggest that estrogen therapy may be useful for treating LBP by limiting and even preventing the progression of IVD degeneration.
Estrogen supplementation alleviates OA
Aside from the spine, OA occurs in many other joints, including the temporomandibular and knee joints (Tables 3 and 4).97 Estrogen has been shown to have important roles in the health and remodeling of joints. For example, OVX resulted in increased thickness of articular soft tissues and decreased bone volume in rats with temporomandibular joint OA.98 In other OVX studies involving the temporomandibular joint, E2/progesterone treatment has been shown to improve the articular disc and condylar cartilaginous layers,99 reduce joint inflammation,100 and alleviate joint nociception.101
OVX monkeys treated with estrogen replacement had less severe OA-related cartilage lesions within the knee joints.102 Similarly, knee cartilage turnover and surface erosion induced by estrogen deficiency were alleviated by estrogen supplementation in rats.82 Moreover, both estrogen and levormeloxifene, a selective estrogen-receptor modulator that inhibits OVX-induced changes, were found to prevent cartilage erosion, and estrogen independently prevented knee cartilage turnover in female OVX rats.61,62 Svetlana et al. demonstrated that OVX surgery led to reductions in serum levels of E2 and C-telopeptide of type II collagen (CTX-II) in rats but that early estrogen therapy could prevent knee cartilage turnover.62 Similarly, tibia bone turnover and the resultant cancellous osteopenia were reduced by E2 treatment in OVX rats.103
Clinical studies have shown that estrogen supplementation produces modest but sustained decreases in joint pain frequency in postmenopausal women. Indeed, joint pain prevalence and severity in postmenopausal patients were lower in those receiving estrogen compared to those receiving placebo, calcium, and vitamin D supplementation.104 Similar findings have been found in other large cross-sectional studies (e.g.,105). Furthermore, knee cartilage volume in postmenopausal women was found to be higher in estrogen therapy users than in nonusers.106 Consistent with these findings, the use of estrogen or estrogen-related (i.e., Prempak-C or Premarin 0.625) therapeutic agents has been associated with a lower prevalence/incidence of knee and hand OA,107 improved knee and hip joint health, and a lower arthroplasty rate.108
Molecular mechanisms by which estrogen alleviates IVD degeneration, FJOA and OA
Despite considerable and growing interest in the topic, the specific mechanisms by which estrogen mitigates LBP and OA remain largely unknown. The role of estrogen in IVD degeneration has been studied the most.17,18,109,110,111,112 There are multiple pathways by which estrogen could impact IVD degeneration, including the nuclear factor kappa-B (NF-κB) signaling pathway, phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, ERβ-p38 mitogen-activated protein kinase (MAPK) signaling pathway, and ER–substance P signaling pathway. Pathways that are most likely involved in OA are the PI3K/AKT pathway, ERα-mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling pathway, and the AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR) signaling pathway (Figs. 1 and 2).
Summary of the potential mechanism by which estrogen alleviates low back pain (LBP) and osteoarthritis (OA). Both inhibition and activation pathways are involved in cell proliferation and viability. Ultimately, LBP may be alleviated by slowing intervertebral disc degeneration (IVDD). The cells involved are nucleus pulposus cells (NPCs), annulus fibrosus cells (AFCs), and cartilage endplate cells (CEPCs). OA may be alleviated by protecting and promoting the proliferation of chondrocytes through the upregulation of mitophagy. AMPK 5’ adenosine monophosphate-activated protein kinase, Col collagen, ER estrogen receptor, MAPK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa-B, PI3K/AKT phosphatidylinositol 3-kinase/protein kinase B
Molecular mechanisms underlying the role of estrogen in LBP
IVD degeneration-related LBP
Pathogenesis of IVD degeneration
The pathogenesis of IVD degeneration is complex and involves multiple factors, including spinal instability, disorganization of the concentric lamellae in the annulus fibrosus, inflammation, matrix degradation, and loss of proteoglycan content in the nucleus pulposus.113,114,115 Within the IVD, the nucleus pulposus, which consists of nucleus pulposus cells and ECM, has been robustly investigated.116,117 Two crucial cellular processes associated with IVD degeneration are aberrant apoptosis and accelerated aging of nucleus pulposus cells. Thus, these processes within nucleus pulposus cells have become an attractive target to prevent and treat IVD degeneration.118,119 Aggrecan and COL2α1 secreted from nucleus pulposus cells are the primary components that maintain the ECM, and their catabolic metabolism is mediated by the family of matrix metalloproteinases (MMPs) and influences the pathological processes of IVD degeneration.120,121,122,123,124
High levels of cytokines are synthesized by resident nucleus pulposus cells during IVD degeneration, including tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β).125 TNFα is a proinflammatory cytokine that influences angiogenesis and nerve ingrowth of the nucleus pulposus.126 IL-1β is another inflammatory cytokine that contributes to the regulation and progression of IVD cell apoptosis.127 Thus, TNF-α and IL-1β have been widely used to induce IVD cell apoptosis in studies.128 As IVD degeneration progresses, other mediators, such as MMP-3 and MMP-13, play particular roles in degrading specific components of the ECM, exacerbating and worsening the condition.129,130,131
Effects of estrogen on the nucleus pulposus during IVD degeneration
In general, E2 prevents ECM degradation by upregulating COL2α1 and aggrecan expression while downregulating MMP-3 and MMP-13 expression in nucleus pulposus cells by regulating redox balance and autophagy.132 Nucleus pulposus cell apoptosis, induced by levofloxacin, has been shown to be reduced in rats treated with E2, which upregulates integrin α2β1, a subtype of the β1 integrin family that can specifically bind to COL2α1.133,134 The effect of 17β-E2 on IL-1β-induced rat nucleus pulposus cell apoptosis has been evaluated extensively in vitro and in vivo. E2 resisted nucleus pulposus cell apoptosis by downregulating MMP-3 and MMP-13 expression and upregulating COL2α1 expression.90,132,134,135 Several lines of evidence suggest that the specific mechanism is related to cellular redox balance.136 First, estrogen deficiency is considered to contribute to the oxidative stress and autophagy that occur in the nucleus pulposus of OVX rats.69 Second, autophagy is thought to protect against oxidative stress.136 Third, E2 restores the redox balance and reduces autophagy levels.69 Fourth, the interaction between 17β-E2 and ERβ was found to attenuate apoptosis (induced by high-glucose conditions) and increase matrix biosynthesis in nucleus pulposus cells in rats by inhibiting oxidative damage.137
NF-κB signaling pathway: The NF-κB family of transcription factors plays critical roles in cell apoptosis and the cellular inflammatory response.138 Nucleus pulposus cell apoptosis can be alleviated by inhibiting the NF-κB signaling pathway through ER-mediated genetic effects.90 Reactive oxygen species (ROS) generation and NF-κB activity in TNF-α-treated nucleus pulposus cells are also inhibited by E2 treatment.138 In this case, TNF-α induced premature senescence of nucleus pulposus cells, but these effects were reversed by E2 treatment through interference with the ROS/NF-κB pathway.138
PI3K/AKT signaling pathway: The PI3K/AKT signaling pathway has important roles in modulating cell function and multiple apoptosis-related proteins, including caspase-3, glycogen synthase kinase-3β (GSK-3β), NF-κB, and mTOR.139,140,141,142 AKT is a serine/threonine protein kinase that can inhibit cell apoptosis via a phosphorylation mechanism dependent on PI3K.143 Increasing evidence points to controlling the PI3K/AKT pathway as a potential therapeutic avenue to prevent and alleviate IVD degeneration.144
(i) PI3K/AKT/mTOR/caspase-3 signaling pathway
It has been demonstrated that apoptosis in human nucleus pulposus cells induced by TNF-α increases caspase-3 protein expression and decreases poly-ADP-ribose polymerase (PARP) expression levels and is alleviated by E2 in a concentration-dependent manner.145 This notion was supported in another study in which E2 was shown to alleviate apoptosis in the same cells (induced by TNF) through upregulation of PARP and downregulation of caspase-3 in the PI3K/AKT pathway.146 Our previous work revealed that E2 treatment effectively abolished the negative effects (including the upregulated COL2α1 and aggrecan and downregulated MMP-3 and MMP-13 expression) caused by IL-1β in rat nucleus pulposus cells by activating the PI3K/AKT/caspase-3 signaling pathways.132 Furthermore, our group has previously explored the downstream signaling pathways of PI3K/Akt and established the pathways by which estrogen protects against apoptosis. By comparing the effects of different antagonists on IL‑1β‑induced apoptosis, including ICI182780 (an ER antagonist), SC75741 (an NF-κB inhibitor), SB216763 (a GSK-3β inhibitor) and rapamycin (an mTOR inhibitor), we revealed that the anti-apoptosis effects of E2 on rat nucleus pulposus cells were reduced by ICI182780 and rapamycin.147 These changes were accompanied by decreases in activated caspase-3 expression and increases in mTOR expression.147 These results highlight the mTOR/caspase‑3 pathway as a potential avenue by which E2 protects nucleus pulposus cells against apoptosis (Fig. 3).109,147
The molecular mechanism underlying the antiapoptotic effect of estrogen on intervertebral disc (IVD) cells. E2 estrogen, PG proteoglycan, COL collagen, MMP matrix metalloproteinase, IL-1β interleukin-1β, TNF-α tumor necrosis factor-α, FOXO3 Forkhead box O-3, P38 MARK p38 mitogen-activated protein kinase. Modified with permission from109. Copyright 2020 Elsevier
(ii) PI3K/AKT/FOXO3 signaling pathway
Forkhead box O-3 (FOXO3) is a transcription factor, and its deficiency can accelerate IVD degeneration.148 It was suggested that matrix loss in human nucleus pulposus cells could be reduced by E2 via the PI3K/AKT/FOXO3 signaling pathway by decreasing MMP-3 levels.135 Specifically, the phosphorylation of FOXO3, caused by E2 induction of the PI3K/AKT pathway, could reduce the activity of the MMP-3 promoter and lead to increased collagen II and aggrecan expression. In addition, both FOXO3 and MMP-3 expression can be increased by the PI3K inhibitor LY294002.135
ERβ-p38 MAPK signaling pathway: The p38 MAPK signaling pathway is important in cell viability and matrix synthesis within the disc nucleus pulposus region, which can be activated by E2.149 The activity of the p38 MAPK pathway in nucleus pulposus cells is increased by E2 and decreased when ERβ function is blocked.150 Together, these findings indicate that E2 increases matrix synthesis within nucleus pulposus cells by activating the ERβ-p38 MAPK pathway and that blocking this pathway might mitigate the positive effects of E2.150
The ER–substance P signaling pathway: Substance P is a neurotransmitter and a modulator of pain perception that acts by altering cellular signaling pathways.151,152,153,154 The relationship between ERs and substance P in nucleus pulposus tissues has been studied in postmenopausal females who underwent posterior lumbar interbody fusion and/or disc excision.53 Findings confirmed that substance P colocalizes with ERα and ERβ both in the cytoplasm and nucleus of the nucleus pulposus cells and that high levels of substance P correlated with low levels of ERα and ERβ expression. That the binding of substance P with neurokinin 1 receptor (NK1R) leads to activation of NF-κB and enhanced production of inflammatory cytokines155,156,157 and that both are reduced in the nucleus pulposus cells of OVX rats in response to estrogen replacement therapy91 suggests that estrogen alleviates nucleus pulposus cell apoptosis by downregulating substance P.91
Effects of estrogen on the annulus fibrosus and cartilage endplate during IVD degeneration
Very few studies have investigated the potential protective effects and underlying mechanisms of E2 treatment on the annulus fibrosus and cartilage endplate. One study reported that IL-1β-induced apoptosis of annulus fibrosus cells is inhibited by 17β-E2 in a dose-dependent manner, which is abolished by an estrogen receptor antagonist.158 In another study, E2 was thought to alleviate rat annulus fibrosus cell apoptosis induced by IL-1β in a time-dependent manner by depressing caspase-3 activity and increasing α1 integrin-mediated adhesion to type I collagen.159 Moreover, transforming growth factor β1 (TGF1) could suppress apoptosis of annulus fibrosus cells by inhibiting excessive autophagy and modulating the PI3K/AKT/mTOR and ERK1/2 signaling pathways.160 Whether estrogen similarly affects the PI3K pathway and inhibits annulus fibrosus cell apoptosis requires further investigation.
Other causes of apoptosis of annulus fibrosus cells include levofloxacin treatment and a high-glucose environment. Levofloxacin is thought to induce these effects, in part, by decreasing the binding of annulus fibrosus cells to type I collagen and upregulating caspase-3, MMP-2, MMP-3, and MMP-13 expression.161,162 High-glucose environments may accelerate annulus fibrosus cell apoptosis by enhancing endoplasmic reticulum stress.163 Whether estrogen protects against annulus fibrosus cell apoptosis evoked by levofloxacin or high-glucose conditions has not been explored.
The cartilage endplate plays a critical role in the metabolic exchange of waste and nutrients in the IVD and thus directly influences the metabolism, proliferation, growth, and apoptosis of nucleus pulposus cells.164,165 Calcification of the cartilage endplate could substantially impair these processes and contribute to the development and maintenance of IVD degeneration.166 The potential for estrogen (or lack thereof) to impact the cartilage endplate has been investigated both in vivo and in vitro. Reduced estrogen levels have been shown to aggravate the cartilage endplate by inducing its calcification, which is mitigated by E2 treatment in a dose-dependent manner.72,167 E2 treatment has been shown to enhance aggrecan and COL2α1 expression and the proliferation of cartilage endplate cells and can inhibit cell apoptosis within the cartilage endplate.167 Furthermore, the effects of E2 could be attenuated by microRNA (miR)-221 or knockdown of ERα, a target of miR-221, which suggests that miR-221 could be involved in the protective effects of E2 on cartilage endplate degeneration.167 Moreover, apoptosis of cells and calcification of the cartilage endplate occurs under oxidative stress conditions (induced by H2O2) via the ROS/p38/ERK/p65 pathway.168 Other conditions/scenarios, such as high-glucose environments and serum deprivation, also promote apoptosis of cartilage endplate cells via mitochondrial mechanisms.169 Serum deprivation increased apoptosis of cartilage endplate cells,170 and this process has been shown to be induced by the mitochondrial intrinsic pathway.171 However, research on the role of estrogen in these processes warrants further exploration.
FJOA-related LBP
FJOA is considered a consequence of spinal degeneration and another potential source of LBP. The specific molecular mechanism of estrogen on FJOA is rarely reported, but we can draw inspiration from some other studies.
Chen et al. reported that increased angiogenesis and neurogenesis were observed in OVX-induced LFJ degeneration.77 In contrast to nondegenerated knee joints, rheumatoid arthritis and OA have higher rates of osteochondral angiogenesis, which is controlled by a proangiogenesis microenvironment in the subchondral bone marrow.172 Fibrovascular replacement of marrow tissue is a process in which new blood vessels form in the subchondral bone, which is the layer of bone just beneath the articular cartilage in joints. This process is associated with the production of various angiogenic cytokines, such as IL-1α, IL-8, IL-10, vascular endothelial growth factor, and platelet-derived growth factor.173,174 Neovascularization and innervation are often closely associated, particularly in the context of joint degeneration and arthritis.175 The colocalization of nerve growth factor with an increased density of nerve fibers in joint degeneration has been identified as a contributing factor to the sensation of arthritis pain by sensitizing the peripheral nerve endings.176 Therefore, it is supposed that estrogen plays important roles in angiogenesis and neurogenesis against FJOA via angiogenic cytokines, which needs further verification. In addition, cartilage is one of the major elements in joint structure. ERα is expressed in different components of the LFJ, including chondrocytes and subchondral bone marrow cells.
Molecular mechanisms underlying the role of estrogen in OA
The accelerated cartilage degeneration and cell apoptosis that occur in people with OA are, at least in part, due to weakened mitophagy in chondrocytes in the articular cartilage.177 Chondrocyte apoptosis and cartilage degeneration are accelerated within the articular cartilage when mitophagy function is impaired in resident chondrocytes.177 As an important inhibitor of autophagy, mTOR is a crucial signaling molecule downstream of AMPK. In addition, the levels of mTOR could be inhibited by an increase in its phosphorylation, promoting autophagy.178 AMPK is a key molecule in chondrocyte metabolism and can regulate metabolism via mediators including mTOR and Sirtuin-1 (SIRT1), an NAD+-dependent type III histone deacetylase.179 SIRT1 plays an important role in protecting chondrocytes, and the apoptosis of chondrocytes could be protected by SIRT1-mediated autophagy.180,181,182 Patients with OA have elevated synovial fluid concentrations of nerve growth factor (NGF),183 which can be amplified by cartilage damage. NGF could upregulate FGF2 (fibroblast growth factor 2) expression in a time- and dose-dependent manner to promote endothelial cell migration and tube formation through the PI3K/Akt and ERK/MAPK pathways in human chondrocytes.184
PI3K/AKT signaling pathway: There is evidence that E2 alleviates OA chondrocyte apoptosis and promotes the proliferation of chondrocytes and the expression of AKT and P-AKT.185 That these effects are reduced by a P-AKT inhibitor further highlights the PI3K/AKT pathway as a potential mechanism by which E2 reduces apoptosis and enhances the proliferation of chondrocytes.185
ERα-MEK-ERK signaling pathway: In a recent study, E2 reduced NGF expression within chondrocytes in both rat knee cartilage and cartilage explants, which could be reversed by a specific ERα inhibitor (MPP). This finding suggests that estrogen downregulated NGF expression via ERα. Furthermore, the induction of NGF expression by TGF-β1 or IL-1β in rat chondrocytes could be decreased by estrogen but increased by PD98059, an inhibitor of the MEK-ERK pathway. This result suggests that the MEK-ERK signaling pathway may be mediated by the modulatory effects of E2 on NGF.186
AMPK/mTOR signaling pathway: Increased levels of mitophagy-related proteins (SIRT1 and p-AMPK) and decreased levels of p-mTOR proteins have been observed in rats following 17β-E2 treatment.180 Other observations in these rats posttreatment included increased mitochondrial autophagosomes and activation of the AMPK/mTOR signaling pathway. These findings provide evidence that E2 may induce mitophagy to protect chondrocytes through the SIRT1-mediated AMPK/mTOR signaling pathway.180
Controversial results and risks associated with estrogen treatments
Although most evidence points to estrogen as a possible therapy to better manage and perhaps even prevent LBP and OA pain, some studies report the opposite results and cannot be neglected. For example, there is prospective evidence that postmenopausal estrogen use is associated with an increased risk of LBP and impaired back function in elderly women.187 Another large study provided cross-sectional evidence that 1 103 women between 55 and 56 years of age receiving hormone replacement therapy had a slightly higher prevalence of LBP than those not receiving hormone replacement therapy.188 Increased risk of chronic LBP has also been found for long-term use of systemic menopausal hormone therapy, especially for the replacement of estrogen.189 Several factors may explain these findings. First, individuals who are more attentive to their symptoms, prone to experiencing pain, and/or experiencing severe climacteric symptoms would be exposed to more opportunities to receive hormone replacement therapy.190 Second, sex steroids and hormone replacement therapy can alter nociceptive processing at various levels of the nervous system, from the peripheral to the spinal and rostral regions of the central nervous system.110 At the peripheral level, estrogen can impact the receptive field properties of primary afferents. At the central level, estrogen can interact with numerous neurochemical systems, such as the endogenous opioid system, which could impact descending pain control.191 Longitudinal trials that test the efficacy of estrogen-related treatments and the underlying mechanisms in clinical populations are needed to address these issues.
Similar observations have been reported in OA. Data from two of the most extensive longitudinal observational studies ever undertaken showed that estrogen treatment did not considerably decrease the radiographic severity of knee or hand OA.107,192,193 That estrogen might differentially impact OA depending on the stage of the condition could explain these results. For instance, early OA is characterized by accelerated bone turnover and consequent bone loss, whereas late-stage OA is characterized by decelerated bone turnover and more subchondral sclerosis.194,195 Thus, estrogen may positively protect against high bone turnover during early OA but prevent the required bone turnover needed to avoid pathological bone thickening during late-stage OA. Moreover, estrogen might contribute differently to specific types of OA. For instance, the role of estrogen within the carpometacarpal joint is unclear.107 Conversely, estrogen has been shown to mediate inflammation in the temporomandibular joint via the NF-kB pathway in a dose-dependent manner.196 Finally, subchondral bone remodeling can result in either an osteoporotic or sclerotic phenotype depending on the ratio between bone formation and resorption. These findings highlight the need to better understand the role of estrogen as OA develops and progresses over time for different joints to more accurately assess if, how and when estrogen interventions will and will not be helpful in preventing and/or managing OA.
In addition, it is important to consider that some diseases and conditions have been associated with estrogen. For instance, a randomized control trial of 16 608 women concluded that the overall health risks exceeded the benefits from the use of estrogen therapies in postmenopausal women.197,198 Some of these risks include venous thromboembolism,199 coronary heart disease and cancer,200 although these are usually reported with long-term (e.g., >5 years) therapy (e.g.,201). As a result, the clinical safety of estrogen replacement therapy has also faced criticism. In response, alternative substances that can mimic estrogen’s effects have gained attention.197 Two examples include selective estrogen receptor modulators (SERMs) and phytoestrogens.202,203,204 SERMs are compounds that interact with estrogen receptors in specific tissues, producing estrogenic effects in some areas but anti-estrogen effects in others.202,205 This selective action takes advantage of the beneficial effects of estrogen, such as preventing bone loss, and limits adverse effects associated with traditional estrogen therapy.202,206 The treatment efficacy of SERMs has been studied in various medical conditions, including breast cancer,207 osteoporosis,208 and cardiovascular disease.209 Raloxifene is an example of a SERM that has been approved by the FDA for the treatment of osteoporosis in postmenopausal women.210 It works by mimicking the positive effects of estrogen on bone density, thereby reducing the risk of fractures.210 Moreover, raloxifene has also shown promise as a prophylactic (preventive) treatment for painful IVD degeneration.210 Although raloxifene and other SERMs offer potential benefits over estrogen therapy, their use should be evaluated on an individual basis. Unlike SERMS, phytoestrogens are naturally occurring plant compounds that have estrogenic activity and are found in various foods, such as soybeans, flaxseeds, and legumes. Phytoestrogens have been investigated for their potential health benefits, including reducing menopausal symptoms,211 improving bone health,212 and providing cardiovascular protection.213
Another critical factor is the dosage of estrogen treatment. On the one hand, estrogen treatment at higher doses (>500 μg·kg−1 per day) has been shown to be more effective at reversing cartilage degeneration than treatment at lower doses (<500 μg·kg−1 per day).214 On the other hand, excess levels of estrogen can lead to autoimmune diseases and cancer, especially breast cancer.200 Further studies are needed to clarify the optimal threshold level of estrogen dosage and the biological mechanisms that drive these positive and/or negative changes, some of which appear to strongly depend on the level of estrogen.
Conclusions
Many studies have reported on the role of estrogen in LBP and OA pain. Of all the estrogen subtypes, E2 is the most potent and is considered to have the most significant role in IVD degeneration, FJOA pain, and OA pain. This review draws on these studies to provide a current update on the relationship between estrogen and LBP/OA pain and the mechanisms that underlie them. Growing evidence continues to reveal critical roles for estrogen in maintaining healthy IVDs, joints and various tissues implicated in LBP and OA. This has opened the door for exploring estrogen as a potential therapeutic option to prevent and manage LBP and OA, especially during low estrogen periods as can occur after menopause. However, the pathways and mechanisms by which estrogen alleviates LBP and OA pain are not yet clear, and the potential side effects of estrogen therapy are still being revealed. Further investigation is warranted to close these knowledge gaps with a focus on exploring which estrogen treatments and at what doses are most effective and safe for different pain conditions at different stages of the condition.
References
Nicolson, P. J. A. et al. Interventions to increase adherence to therapeutic exercise in older adults with low back pain and/or hip/knee osteoarthritis: a systematic review and meta-analysis. Br. J. Sports Med. 51, 791–799 (2017).
Fatoye, F., Gebrye, T. & Odeyemi, I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol. Int. 39, 619–626 (2019).
Will, J. S., Bury, D. C. & Miller, J. A. Mechanical low back pain. Am. Fam. Physician 98, 421–428 (2018).
Jiang, J. W. et al. Up-regulation of TRAF2 inhibits chondrocytes apoptosis in lumbar facet joint osteoarthritis. Biochem. Biophys. Res. Co. 503, 1659–1665 (2018).
Diwan, A. D. & Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 6, e1231 (2023).
Hoy, D. et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 64, 2028–2037 (2012).
Hunter, D. J., McDougall, J. J. & Keefe, F. J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. North Am. 34, 623–643 (2008).
Wu, A. et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 8, 299 (2020).
Wáng, Y. X., Wáng, J. Q. & Káplár, Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quant. Imaging Med. Surg. 6, 199 (2016).
Dulay, G. S., Cooper, C. & Dennison, E. M. Knee pain, knee injury, knee osteoarthritis & work. Best. Pr. Res. Clin. Rheumatol. 29, 454–461 (2015).
Chidi-Ogbolu, N. & Baar, K. Effect of estrogen on musculoskeletal performance and injury risk. Front. Physiol. 9, 1834 (2018).
Richardson, H. et al. Baseline estrogen levels in postmenopausal women participating in the MAP.3 breast cancer chemoprevention trial. Menopause 27, 693–700 (2020).
Dedicação, A. C. et al. Prevalence of musculoskeletal pain in climacteric women of a Basic Health Unit in São Paulo/SP. Rev. dor 18, 212–216 (2017).
Gibson, C. J., Li, Y., Bertenthal, D., Huang, A. J. & Seal, K. H. Menopause symptoms and chronic pain in a national sample of midlife women veterans. Menopause 26, 708–713 (2019).
Klyne, D. M. et al. Cohort profile: why do people keep hurting their back? BMC Res. Notes 13, 538 (2020).
Klyne, D. M., Barbe, M. F., James, G. & Hodges, P. W. Does the interaction between local and systemic inflammation provide a link from psychology and lifestyle to tissue health in musculoskeletal conditions? Int. J. Mol. Sci. 22, 7299 (2021).
Vergroesen, P. P. A. et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr. Cartil. 23, 1057–1070 (2015).
Millecamps, M. & Stone, L. S. Delayed onset of persistent discogenic axial and radiating pain after a single-level lumbar intervertebral disc injury in mice. Pain 159, 1843–1855 (2018).
Erwin, W. M. & Hood, K. E. The cellular and molecular biology of the intervertebral disc: a clinician’s primer. J. Can. Chiropr. Assoc. 58, 246–257 (2014).
Ghannam, M. et al. Surgical anatomy, radiological features, and molecular biology of the lumbar intervertebral discs. Clin. Anat. 30, 251–266 (2017).
De Geer, C. M. Intervertebral disk nutrients and transport mechanisms in relation to disk degeneration: a narrative literature review. J. Chiropr. Med. 17, 97–105 (2018).
Kim, J. H., Ham, C. H. & Kwon, W. K. Current knowledge and future therapeutic prospects in symptomatic intervertebral disc degeneration. Yonsei Med. J. 63, 199–210 (2022).
Oichi, T., Taniguchi, Y., Oshima, Y., Tanaka, S. & Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 3, e1076 (2020).
Gruber, H. E. & Hanley, E. N. Analysis of aging and degeneration of the human intervertebral disc-Comparison of surgical specimens with normal controls. Spine 23, 751–757 (1998).
Hasty, P., Campisi, J., Hoeijmakers, J., van Steeg, H. & Vijg, J. Aging and genome maintenance: lessons from the mouse? Science 299, 1355–1359 (2003).
Sadowska, A. et al. Inflammaging in cervical and lumbar degenerated intervertebral discs: analysis of proinflammatory cytokine and TRP channel expression. Eur. Spine J. 27, 564–577 (2018).
latridis, J. C., Godburn, K., Wuertz, K., Alini, M. & Roughley, P. J. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine 36, 203–209 (2011).
Gellhorn, A. C., Katz, J. N. & Suri, P. Osteoarthritis of the spine: the facet joints. Nat. Rev. Rheumatol. 9, 216–224 (2013).
Suri, P., Hunter, D. J., Rainville, J., Guermazi, A. & Katz, J. N. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthr. Cartil. 21, 1199–1206 (2013).
Morris, M., Pellow, J., Solomon, E. M. & Tsele-Tebakang, T. Physiotherapy and a homeopathic complex for chronic low-back pain due to osteoarthritis: a randomized, controlled pilot study. Alter. Ther. Health Med. 22, 48–56 (2016).
Lindsey, T. & Dydyk, A. M. in StatPearls (2023).
O’Leary, S. A. et al. Facet joints of the spine: structure-function relationships, problems and treatments, and the potential for regeneration. Annu. Rev. Biomed. Eng. 20, 145–170 (2018).
Caelers, I. J. et al. Lumbar intervertebral motion analysis during flexion and extension cinematographic recordings in healthy male participants: protocol. JMIR Res. Protoc. 9, e14741 (2020).
Vina, E. R. & Kwoh, C. K. Epidemiology of osteoarthritis: literature update. Curr. Opin. Rheumatol. 30, 160–167 (2018).
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 5, 16044 (2017).
Alpay-Kanitez, N., Celik, S. & Bes, C. Polyarthritis and its differential diagnosis. Eur. J. Rheumatol. 6, 167–173 (2019).
Prieto-Alhambra, D. et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 73, 1659–1664 (2014).
Murphy, L. B. et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthr. Cartil. 18, 1372–1379 (2010).
Losina, E. et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 65, 703–711 (2013).
Glass, N. et al. Examining sex differences in knee pain: the multicenter osteoarthritis study. Osteoarthr. Cartil. 22, 1100–1106 (2014).
Belachew, E. B. & Sewasew, D. T. Molecular mechanisms of endocrine resistance in estrogen-positive breast cancer. Front. Endocrinol. 12, 599586 (2021).
Cauley, J. A. Estrogen and bone health in men and women. Steroids 99, 11–15 (2015).
Nilsson, S. & Gustafsson, J. Å. Estrogen receptors: therapies targeted to receptor subtypes. Clin. Pharm. Ther. 89, 44–55 (2011).
Pöllänen, E. et al. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre‐ and postmenopausal women. Aging Cell 10, 650–660 (2011).
Ali, E. S., Mangold, C. & Peiris, A. N. Estriol: emerging clinical benefits. Menopause 24, 1081–1085 (2017).
Kumar, R. S. & Goyal, N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. 269, 119091 (2021).
Emmanuelle, N. E. et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int. J. Mol. Sci. 22, 1568 (2021).
Khosla, S., Oursler, M. J. & Monroe, D. G. Estrogen and the skeleton. Trends Endocrinol. Metab. 23, 576–581 (2012).
Levin, E. R. Extranuclear steroid receptors are essential for steroid hormone actions. Annu. Rev. Med. 66, 271–280 (2015).
Roman-Blas, J. A., Castaneda, S., Largo, R. & Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 11, 241 (2009).
Song, X. X. et al. Estrogen receptor expression in lumbar intervertebral disc of the elderly: gender- and degeneration degree-related variations. Jt. Bone Spine 81, 250–253 (2014).
Song, X. X., Shi, S., Guo, Z., Li, X. F. & Yu, B. W. Estrogen receptors involvement in intervertebral discogenic pain of the elderly women: Colocalization and correlation with the expression of Substance P in nucleus pulposus. Oncotarget 8, 38136–38144 (2017).
Wei, A. Q. et al. Expression and functional roles of estrogen receptor GPR30 in human intervertebral disc. J. Steroid Biochem. 158, 46–55 (2016).
Dreier, R., Ising, T., Ramroth, M. & Rellmann, Y. Estradiol inhibits ER stress-induced apoptosis in chondrocytes and contributes to a reduced osteoarthritic cartilage degeneration in female mice. Front. Cell Dev. Biol. 10, 913118 (2022).
Braidman, I. P. et al. Localization of estrogen receptor β protein expression in adult human bone. J. Bone Min. Res. 16, 214–220 (2001).
Dietrich, W. et al. Estrogen Receptor-β Is the Predominant Estrogen Receptor Subtype in Normal Human Synovia. J. Soc. Gynecol. Investig. 13, 512–517 (2006).
Hang, X. et al. Estrogen Protects Articular Cartilage by Downregulating ASIC1a in Rheumatoid Arthritis. J. Inflamm. Res. 14, 843–858 (2021).
Maneix, L. et al. 17Beta-oestradiol up-regulates the expression of a functional UDP-glucose dehydrogenase in articular chondrocytes: comparison with effects of cytokines and growth factors. Rheumatology. 47, 281–288 (2008).
Claassen, H., Schünke, M. & Kurz, B. Estradiol protects cultured articular chondrocytes from oxygen-radical-induced damage. Cell Tissue Res. 319, 439–445 (2005).
Christgau, S. et al. Suppression of elevated cartilage turnover in postmenopausal women and in ovariectomized rats by estrogen and a selective estrogen-receptor modulator (SERM). Menopause 11, 508–518 (2004).
Oestergaard, S. et al. Effects of ovariectomy and estrogen therapy on type II collagen degradation and structural integrity of articular cartilage in rats: implications of the time of initiation. Arthritis Rheum. 54, 2441–2451 (2006).
SILBERBERG, M. & SILBERBERG, R. Modifying action of estrogen on the evolution of osteoarthrosis in mice of different ages. Endocrinology 72, 449–451 (1963).
Tsai, C. L. & Liu, T. K. Estradiol-induced knee osteoarthrosis in ovariectomized rabbits. Clin. Orthop. Relat. Res. 291, 295–302 (1993).
Rosner, I. A. et al. Pathologic and metabolic responses of experimental osteoarthritis to estradiol and an estradiol antagonist. Clin. Orthop. Relat. Res. 171, 280–286 (1982).
Ha, K. Y. et al. Expression of estrogen receptor of the facet joints in degenerative spondylolisthesis. Spine 30, 562–566 (2005).
Wang, T., Zhang, L., Huang, C., Cheng, A. G. & Dang, G. T. Relationship between osteopenia and lumbar intervertebral disc degeneration in ovariectomized rats. Calcif. Tissue Int. 75, 205–213 (2004).
Liu, Q. et al. Estrogen deficiency exacerbates intervertebral disc degeneration induced by spinal instability in rats. Spine 44, E510–E519 (2019).
Jin, L. Y. et al. Estradiol alleviates intervertebral disc degeneration through modulating the antioxidant enzymes and inhibiting autophagy in the model of menopause rats. Oxid. Med. Cell Longev. 2018, 7890291 (2018).
Jia, H. et al. Oestrogen and parathyroid hormone alleviate lumbar intervertebral disc degeneration in ovariectomized rats and enhance Wnt/β-catenin pathway activity. Sci. Rep. 6, 27521 (2016).
Xiao, Z. F. et al. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res. Ther. 20, 207 (2018).
Sheng, B. et al. Protective effect of estrogen against calcification in the cartilage endplate. Int. J. Clin. Exp. Pathol. 11, 1660–1666 (2018).
Shelby, T. et al. The role of sex hormones in degenerative disc disease. Global Spine J, 21925682231152826 (2023).
Kupka, J. et al. Adrenoceptor expression during intervertebral disc degeneration. Int. J. Mol. Sci. 21, 2085 (2020).
Wu, T. et al. Three-dimensional visualization and pathologic characteristics of cartilage and subchondral bone changes in the lumbar facet joint of an ovariectomized mouse model. Spine J. 18, 663–673 (2018).
Gou, Y. et al. Salmon calcitonin attenuates degenerative changes in cartilage and subchondral bone in lumbar facet joint in an experimental rat model. Med. Sci. Monit. 24, 2849–2857 (2018).
Chen, H., Zhu, H., Zhang, K., Chen, K. & Yang, H. Estrogen deficiency accelerates lumbar facet joints arthritis. Sci. Rep. 7, 1379 (2017).
Zhao, Y. et al. Lumbar disk degeneration in female patients with and without ovariectomy: a case-control study. World Neurosurg. 156, 68–75 (2021).
Ketut, I. Suyasa*, I. G. N. Y. S. The role of aging, body mass index and estrogen on symptomatic lumbar osteoarthritis in post-menopausal women. Int. J. Res. Med. Sci. 4, 1325 (2016).
Xu, X. et al. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med. Sci. Monit. 25, 3146–3153 (2019).
Jiang, A. et al. Phenotype changes of subchondral plate osteoblasts based on a rat model of ovariectomy-induced osteoarthritis. Ann. Transl. Med. 8, 476 (2020).
Høegh-Andersen, P. et al. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res. Ther. 6, R169–R180 (2004).
Hashem, G. et al. Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss. Arthritis Res. Ther. 8, R98 (2006).
Turner, A. S., Athanasiou, K. A., Zhu, C. F., Alvis, M. R. & Bryant, H. U. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthr. Cartil. 5, 63–69 (1997).
Dai, G., Wang, S., Li, J., Liu, C. & Liu, Q. The validity of osteoarthritis model induced by bilateral ovariectomy in guinea pig. J. Huazhong Univ. Sci. Technol. Med. Sci. 26, 716–719 (2006).
Sniekers, Y. H., Weinans, H., Bierma-Zeinstra, S. M., van Leeuwen, J. P. & van Osch, G. J. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment - a systematic approach. Osteoarthr. Cartil. 16, 533–541 (2008).
Lu, Z. H., Zhang, A. H., Wang, J. C., Han, K. J. & Gao, H. Estrogen alleviates post-traumatic osteoarthritis progression and decreases p-EGFR levels in female mouse cartilage. BMC Musculoskelet. Disord. 23, 685 (2022).
Srikanth, V. K. et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 13, 769–781 (2005).
Liu, S. et al. 17β-Estradiol inhibits intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in a rat model. Artif. Cells Nanomed. Biotechnol. 46, 182–191 (2018).
Wang, H. et al. 17β-Estradiol alleviates intervertebral disc degeneration by inhibiting NF-κB signal pathway. Life Sci. 284, 119874 (2021).
Song, X. X., Jin, L. Y., Li, X. F., Luo, Y. & Yu, B. W. Substance P Mediates Estrogen Modulation Proinflammatory Cytokines Release in Intervertebral Disc. Inflammation 44, 506–517 (2020).
Baron, Y. M., Brincat, M. P., Galea, R. & Calleja, N. Intervertebral disc height in treated and untreated overweight post-menopausal women. Hum. Reprod. 20, 3566–3570 (2005).
Marty-Poumarat, C., Scattin, L., Marpeau, M., Garreau De Loubresse, C. & Aegerter, P. Natural history of progressive adult scoliosis. Spine 32, 1227–1234 (2007).
Marty-Poumarat, C. et al. Does hormone replacement therapy prevent lateral rotatory spondylolisthesis in postmenopausal women? Eur. Spine J. 21, 1127–1134 (2011).
Muscat Baron, Y., Brincat, M. P., Galea, R. & Calleja, N. Low intervertebral disc height in postmenopausal women with osteoporotic vertebral fractures compared to hormone-treated and untreated postmenopausal women and premenopausal women without fractures. Climacteric 10, 314–319 (2007).
Park, J. H., Hong, J. Y., Han, K., Han, S. W. & Chun, E. M. Relationship between hormone replacement therapy and spinal osteoarthritis: a nationwide health survey analysis of the elderly Korean population. BMJ Open 7, e018063 (2017).
Cheung, P., Gossec, L. & Dougados, M. What are the best markers for disease progression in osteoarthritis (OA)? Best. Pr. Res. Clin. Rheumatol. 24, 81–92 (2010).
Yasuoka, T., Nakashima, M., Okuda, T. & Tatematsu, N. Effect of estrogen replacement on temporomandibular joint remodeling in ovariectomized rats. J. Oral. Maxillofac. Surg. 58, 189–196 (2000).
Abdrabuh, A., Baljon, K. & Alyami, Y. Impact of estrogen therapy on temporomandibular joints of rats: Histological and hormone analytical study. Saudi Dent. J. 33, 608–613 (2021).
Torres-Chávez, K. E. et al. Effect of gonadal steroid hormones on formalin-induced temporomandibular joint inflammation. Eur. J. Pain. 16, 204–216 (2011).
Fischer, L. et al. The Influence of Sex and Ovarian Hormones on Temporomandibular Joint Nociception in Rats. J. Pain. 9, 630–638 (2008).
Ham, K. D., Loeser, R. F., Lindgren, B. R. & Carlson, C. S. Effects of long‐term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum. 46, 1956–1964 (2002).
Schmidt, I. U., Wakley, G. K. & Turner, R. T. Effects of Estrogen and Progesterone on Tibia Histomorphometry in Growing Rats. Calcif. Tissue Int. 67, 47–52 (2000).
Chlebowski, R. T. et al. Estrogen alone and joint symptoms in the Women’s Health Initiative randomized trial. Menopause 25, 1313 (2018).
Jung, J. H. et al. Knee osteoarthritis and menopausal hormone therapy in postmenopausal women: a nationwide cross-sectional study. Menopause 26, 598–602 (2018).
Wluka, A. E., Davis, S. R., Bailey, M., Stuckey, S. & Cicuttini, F. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann. Rheum. Dis. 60, 332–336 (2001).
Spector, T. D., Nandra, D., Hart, D. J. & Doyle, D. V. Is hormone replacement therapy protective for hand and knee osteoarthritis in women?: The Chingford study. Ann. Rheum. Dis. 56, 432–434 (1997).
Cirillo, D. J., Wallace, R. B., Wu, L. & Yood, R. A. Effect of hormone therapy on risk of hip and knee joint replacement in the Women’s Health Initiative. Arthritis Rheum. 54, 3194–3204 (2006).
Yang, S. D., Zhang, F., Ma, J. T. & Ding, W. Y. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res. Rev. 57, 100978 (2020).
Chen, Q., Zhang, W., Sadana, N. & Chen, X. Estrogen receptors in pain modulation: cellular signaling. Biol. Sex. Differ. 12, 22 (2021).
Sun, L. H. et al. Estrogen modulation of visceral pain. J. Zhejiang Univ. Sci. B 20, 628–636 (2019).
Xunlu, Y. et al. Integrative bioinformatics analysis reveals potential gene biomarkers and analysis of function in human degenerative disc annulus fibrosus cells. Biomed. Res. Int. 2019, 9890279 (2019).
Huang, Y. C., Urban, J. P. & Luk, K. D. Intervertebral disc regeneration: do nutrients lead the way? Nat. Rev. Rheumatol. 10, 561–566 (2014).
Grunhagen, T. D., Shirazi-Adl, A., Fairbank, J. C. & Urban, J. P. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop. Clin. North Am. 42, 465–477 (2011).
Stephan, S., Johnson, W. E. & Roberts, S. The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur. Cell Mater. 22, 97–108 (2011).
Adams, M. A., Dolan, P. & McNally, D. S. The internal mechanical functioning of intervertebral discs and articular cartilage, and its relevance to matrix biology. Matrix Biol. 28, 384–389 (2009).
Zhang, F. A. N., Zhao, X., Shen, H. & Zhang, C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int. J. Mol. Med. 37, 1439–1448 (2016).
Zhou, G. Q., Yang, F., Leung, V. V. L. & Cheung, K. M. C. (v) Molecular and cellular biology of the intervertebral disc and the use of animal models. Curr. Orthop. 22, 267–273 (2008).
Le Maitre, C. L., Freemont, A. J. & Hoyland, J. A. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 9, R45 (2007).
Roberts, S., Evans, H., Trivedi, J. & Menage, J. Histology and pathology of the human intervertebral disc. J. Bone Jt. Surg. Am. 88, 10–14 (2006).
Kepler, C. K. M. D. M. B. A., Ponnappan, R. K. M. D., Tannoury, C. A. M. D., Risbud, M. V. P. & Anderson, D. G. M. D. The molecular basis of intervertebral disc degeneration. Spine J. 13, 318–330 (2013).
Xu, Y. Q., Zhang, Z. H., Zheng, Y. F. & Feng, S. Q. Dysregulated miR-133a mediates loss of type ii collagen by directly targeting matrix metalloproteinase 9 (mmp9) in human intervertebral disc degeneration. Spine 41, E717–E724 (2016).
Zawilla, N. H. et al. Matrix metalloproteinase-3, vitamin D receptor gene polymorphisms, and occupational risk factors in lumbar disc degeneration. J. Occup. Rehabil. 24, 370–381 (2013).
Molinos, M. et al. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 12, 20150429 (2015).
Lee, S. et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin. Biochem. 42, 1504–1511 (2009).
Currò, M. et al. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral. Dis. 20, 616–623 (2014).
Le Maitre, C. L., Freemont, A. J. & Hoyland, J. A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 7, R732–R745 (2005).
Johnson, Z. I., Schoepflin, Z. R., Choi, H., Shapiro, I. M. & Risbud, M. V. Disc in flames: Roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur. Cell Mater. 30, 104–117 (2015).
Gu, S. X. et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene 555, 80–87 (2015).
Mavrogonatou, E., Angelopoulou, M. T. & Kletsas, D. The catabolic effect of TNFα on bovine nucleus pulposus intervertebral disc cells and the restraining role of glucosamine sulfate in the TNFα-mediated up-regulation of MMP-3. J. Orthop. Res. 32, 1701–1707 (2014).
Yao, Z. et al. Salubrinal Suppresses IL-17-Induced Upregulation of MMP-13 and Extracellular Matrix Degradation Through the NF-kB Pathway in Human Nucleus Pulposus Cells. Inflammation 39, 1997–2007 (2016).
Yang, S. D., Ma, L., Yang, D. L. & Ding, W. Y. Combined effect of 17 beta-estradiol and resveratrol against apoptosis induced by interleukin-1 beta in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ 4, e1640 (2016).
Song, M. et al. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed. Pharmacother. 84, 1137–1143 (2016).
Yang, S. D. et al. 17β-Estradiol protects against apoptosis induced by interleukin-1β in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis 20, 348–357 (2015).
Gao, X. W., Su, X. T., Lu, Z. H. & Ou, J. 17β-Estradiol prevents extracellular matrix degradation by downregulating MMP3 expression via PI3K/Akt/FOXO3 pathway. Spine 45, 292–299 (2020).
Shen, C., Yan, J., Jiang, L. S. & Dai, L. Y. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res. Ther. 13, R132 (2011).
Yang, D. et al. 17β-Estradiol/extrogen receptor β alleviates apoptosis and enhances matrix biosynthesis of nucleus pulposus cells through regulating oxidative damage under a high glucose condition. Biomed. Pharmacother. 107, 1004–1009 (2018).
Li, P. et al. 17beta-estradiol attenuates TNF-alpha-induced premature senescence of nucleus pulposus cells through regulating the ROS/NF-kappaB pathway. Int. J. Biol. Sci. 13, 145–156 (2017).
Lin, C. Y., Chen, J. H., Fu, R. H. & Tsai, C. W. Induction of Pi form of glutathione S‑transferase by carnosic acid is mediated through PI3K/Akt/NF-κB pathway and protects against neurotoxicity. Chem. Res. Toxicol. 27, 1958–1966 (2014).
Huang, H., Zhong, R., Xia, Z., Song, J. & Feng, L. Neuroprotective effects of rhynchophylline against ischemic brain injury via regulation of the Akt/mTOR and TLRs signaling pathways. Molecules 19, 11196–11210 (2014).
Kim, D. E., Kim, B., Shin, H. S., Kwon, H. J. & Park, E. S. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp. Cell Res. 327, 264–275 (2014).
Musumeci, G. et al. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int. J. Mol. Sci. 14, 15767–15784 (2013).
Cravero, J. D. et al. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin‐like growth factor 1–mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 60, 492–500 (2009).
Guo, F., Zou, Y. & Zheng, Y. Moracin M inhibits lipopolysaccharide-induced inflammatory responses in nucleus pulposus cells via regulating PI3K/Akt/mTOR phosphorylation. Int. Immunopharmacol. 58, 80–86 (2018).
Liu, H. et al. Protective role of 17β-estradiol on tumor necrosis factor-α-induced apoptosis in human nucleus pulposus cells. Mol. Med. Rep. 16, 1093–1100 (2017).
Wang, T. et al. 17β-Estradiol inhibites tumor necrosis factor-α induced apoptosis of human nucleus pulposus cells via the PI3K/Akt pathway. Med. Sci. Monit. 22, 4312–4322 (2016).
Guo, H. T. et al. 17beta‑Estradiol protects against interleukin‑1beta‑induced apoptosis in rat nucleus pulposus cells via the mTOR/caspase‑3 pathway. Mol. Med. Rep. 20, 1523–1530 (2019).
Kim, J. et al. Syringaresinol reverses age-related skin atrophy by suppressing FoxO3a-mediated matrix metalloproteinase–2 activation in Copper/Zinc superoxide dismutase–deficient mice. J. Investig. Dermatol. 139, 648–655 (2019).
Li, P. et al. Osmolarity affects matrix synthesis in the nucleus pulposus associated with the involvement of MAPK pathways: A study of ex vivo disc organ culture system. J. Orthop. Res. 34, 1092–1100 (2016).
Li, P. et al. Estrogen enhances matrix synthesis in nucleus pulposus cell through the estrogen receptor β-p38 MAPK pathway. Cell Physiol. Biochem. 39, 2216–2226 (2016).
Lisowska, B., Lisowski, A. & Siewruk, K. Substance P and chronic pain in patients with chronic inflammation of connective tissue. PLoS One 10, e0139206 (2015).
Wu, S. et al. Occurrence of substance P and neurokinin receptors during the early phase of spinal fusion. Mol. Med. Rep. 17, 6691–6696 (2018).
Zieglgansberger, W. Substance P and pain chronicity. Cell Tissue Res. 375, 227–241 (2019).
Zheng, J. et al. Reactive oxygen species mediate low back pain by upregulating substance P in intervertebral disc degeneration. Oxid. Med. Cell Longev. 2021, 6681815 (2021).
Martinez, A. N. & Philipp, M. T. Substance P and antagonists of the neurokinin-1 receptor in neuroinflammation associated with infectious and neurodegenerative diseases of the central nervous system. J. Neurol. Neuromed. 1, 29–36 (2016).
Ho, W. Z. & Douglas, S. D. Substance P and neurokinin-1 receptor modulation of HIV. J. Neuroimmunol. 157, 48–55 (2004).
Bost, K. L. Tachykinin-mediated modulation of the immune response. Front. Biosci. 9, 3331–3332 (2004).
Wang, H. et al. Different concentrations of 17β-estradiol modulates apoptosis induced by interleukin-1β in rat annulus fibrosus cells. Mol. Med. Rep. 10, 2745–2751 (2014).
Zhao, C. M. et al. 17β-Estradiol protects rat annulus fibrosus cells against apoptosis via α1 integrin-mediated adhesion to type I collagen: an in-vitro study. Med. Sci. Monit. 22, 1375–1383 (2016).
Ni, B. B. et al. The effect of transforming growth factor beta1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine 70, 87–96 (2014).
Bai, Z. L. et al. Toxic effects of levofloxacin on rat annulus fibrosus cells: an in-vitro study. Med. Sci. Monit. 20, 2205–2212 (2014).
Wei, H. K. et al. Levofloxacin increases apoptosis of rat annulus fibrosus cells via the mechanism of upregulating MMP-2 and MMP-13. Int. J. Clin. Exp. Med. 8, 20198–20207 (2015).
Pang, L., Yang, K. & Zhang, Z. High-glucose environment accelerates annulus fibrosus cell apoptosis by regulating endoplasmic reticulum stress. Biosci. Rep. 40, BSR20200262 (2020).
Madiraju, P., Gawri, R., Wang, H., Antoniou, J. & Mwale, F. Mechanism of parathyroid hormone-mediated suppression of calcification markers in human intervertebral disc cells. Eur. Cell Mater. 25, 268–283 (2013).
Yang, S. D. et al. 17β-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis 19, 789–800 (2014).
Peng, B. & Li, Y. Concerns about cell therapy for intervertebral disc degeneration. NPJ Regen. Med. 7, 46 (2022).
Sheng, B. et al. Protective effect of estrogen against intervertebral disc degeneration is attenuated by miR-221 through targeting estrogen receptor α. Acta Biochim. Biophys. Sin. 50, 345–354 (2018).
Han, Y. et al. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-kappaB pathway: implications for disc degeneration. Biochem. Biophys. Res. Commun. 516, 1026–1032 (2019).
Jiang, Z. et al. High glucose-induced excessive reactive oxygen species promote apoptosis through mitochondrial damage in rat cartilage endplate cells. J. Orthop. Res. 36, 2476–2483 (2018).
Ding, L. et al. Effects of CCN3 on rat cartilage endplate chondrocytes cultured under serum deprivation in vitro. Mol. Med. Rep. 13, 2017–2022 (2016).
Li, D. et al. Role of the mitochondrial pathway in serum deprivation-induced apoptosis of rat endplate cells. Biochem. Biophys. Res. Commun. 452, 354–360 (2014).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 49, 1852–1861 (2010).
Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 4, 221–229 (2019).
Yuan, X. L. et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 22, 1077–1089 (2014).
Grassel, S., Zaucke, F. & Madry, H. Osteoarthritis: novel molecular mechanisms increase our understanding of the disease pathology. J. Clin. Med. 10, 1938 (2021).
Aso, K. et al. Time course and localization of nerve growth factor expression and sensory nerve growth during progression of knee osteoarthritis in rats. Osteoarthr. Cartil. 30, 1344–1355 (2022).
Rockel, J. S. & Kapoor, M. Autophagy: controlling cell fate in rheumatic diseases. Nat. Rev. Rheumatol. 13, 193 (2017).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. cell 30, 214–226 (2008).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Mei, R. et al. 17β-Estradiol induces mitophagy upregulation to protect chondrocytes via the SIRT1-mediated AMPK/mTOR signaling pathway. Front. Endocrinol. (Lausanne) 11, 615250 (2021).
Matsuzaki, T. et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann. Rheum. Dis. 73, 1397–1404 (2014).
Zhang, Z. et al. Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1‐autophagy pathway. J. Cell Mol. Med. 23, 177–193 (2019).
Montagnoli, C. et al. β-NGF and β-NGF receptor upregulation in blood and synovial fluid in osteoarthritis. Biol. Chem. 398, 1045–1054 (2017).
Yu, X. et al. NGF increases FGF2 expression and promotes endothelial cell migration and tube formation through PI3K/Akt and ERK/MAPK pathways in human chondrocytes. Osteoarthr. Cartil. 27, 526–534 (2019).
Huang, J. G. et al. 17β-Estradiol promotes cell proliferation in rat osteoarthritis model chondrocytes via PI3K/Akt pathway. Cell Mol. Biol. Lett. 16, 564–575 (2011).
Shang, X., Zhang, L., Jin, R., Yang, H. & Tao, H. Estrogen regulation of the expression of pain factor NGF in rat chondrocytes. J. Pain. Res. 14, 931–940 (2021).
Musgrave, D. S., Vogt, M. T., Nevitt, M. C. & Cauley, J. A. Back problems among postmenopausal women taking estrogen replacement therapy: The study of osteoporotic fractures. Spine 26, 1606–1612 (2001).
Brynhildsen, J. O., Björs, E., Skarsgård, C. & Hammar, M. L. Is hormone replacement therapy a risk factor for low back pain among postmenopausal women? Spine 23, 809–813 (1998).
Heuch, I., Heuch, I., Hagen, K., Storheim, K. & Zwart, J. A. Menopausal hormone therapy, oral contraceptives and risk of chronic low back pain: the HUNT Study. BMC Musculoskelet. Disord. 24, 84 (2023).
Ortmann, O. & Lattrich, C. The treatment of climacteric symptoms. Dtsch Arztebl Int. 109, 316–323 (2012). quiz 324.
Smith, Y. R. et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J. Neurosci. 26, 5777–5785 (2006).
Hannan, M. T., Felson, D. T., Anderson, J. J., Naimark, A. & Kannel, W. B. Estrogen use and radiographic osteoarthritis of the knee in women. The Framingham Osteoarthritis Study. Arthritis Rheum. 33, 525–532 (1990).
Zhang, Y. et al. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study. Arthritis Rheum. 41, 1867–1873 (1998).
Kwan Tat, S., Lajeunesse, D., Pelletier, J. P. & Martel-Pelletier, J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best. Pr. Res Clin. Rheumatol. 24, 51–70 (2010).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Kou, X. X. et al. 17beta-estradiol aggravates temporomandibular joint inflammation through the NF-kappaB pathway in ovariectomized rats. Arthritis Rheum. 63, 1888–1897 (2011).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Miller, V. M. & Harman, S. M. An update on hormone therapy in postmenopausal women: mini-review for the basic scientist. Am. J. Physiol.-Heart C. 313, H1013–H1021 (2017).
Canonico, M. et al. Hormone therapy and venous thromboembolism among postmenopausal women - Impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation 115, 840–845 (2007).
Patel, S., Homaei, A., Raju, A. B. & Meher, B. R. Estrogen: the necessary evil for human health, and ways to tame it. Biomed. Pharmacother. 102, 403–411 (2018).
LaCroix, A. Z. et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial EDITORIAL COMMENT. Obstet. Gynecol. Surv. 66, 427–429 (2011).
Goldstein, S. R. Selective estrogen receptor modulators and bone health. Climacteric 25, 56–59 (2022).
Desmawati, D. & Sulastri, D. Phytoestrogens and their health effect. Open Access Maced. J. Med Sci. 7, 495–499 (2019).
Li, X. et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine 33, 2586–2595 (2008).
Lewis, J. S. & Jordan, V. C. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat. Res. 591, 247–263 (2005).
Lugo, L., Villalvilla, A., Largo, R., Herrero-Beaumont, G. & Roman-Blas, J. A. Selective estrogen receptor modulators (SERMs): new alternatives for osteoarthritis? Maturitas 77, 380–384 (2014).
Downton, T., Zhou, F., Segara, D., Jeselsohn, R. & Lim, E. Oral selective estrogen receptor degraders (SERDs) in breast cancer: advances, challenges, and current status. Drug Des. Devel. Ther. 16, 2933–2948 (2022).
Deng, Y., Li, L., Li, C., Wang, F. & Qu, Y. Efficacy of combined medication of risedronate sodium and selective estrogen receptor modulator on the postmenopausal osteoporosis. Pak. J. Pharm. Sci. 33, 495–498 (2020).
Wilcox, N. S. et al. Sex-specific cardiovascular risks of cancer and its therapies. Circ. Res. 130, 632–651 (2022).
Bhadouria, N., Berman, A. G., Wallace, J. M. & Holguin, N. Raloxifene stimulates estrogen signaling to protect against age- and sex-related intervertebral disc degeneration in mice. Front. Bioeng. Biotechnol. 10, 924918 (2022).
Chen, M. N., Lin, C. C. & Liu, C. F. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric 18, 260–269 (2015).
Lagari, V. S. & Levis, S. Phytoestrogens and bone health. Curr. Opin. Endocrinol. Diabetes Obes. 17, 546–553 (2010).
Chudzinska, M. et al. Resveratrol and cardiovascular system-the unfulfilled hopes. Ir. J. Med Sci. 190, 981–986 (2021).
Gilmer, G. et al. Uncovering the “riddle of femininity” in osteoarthritis: a systematic review and meta-analysis of menopausal animal models and mathematical modeling of estrogen treatment. Osteoarthr. Cartil. 31, 447–457 (2023).
Kyllönen, E. S., Väänänen, H. K., Vanharanta, J. H. V. & Heikkinen, J. E. Influence of estrogen-progestin treatment on back pain and disability among slim premenopausal women with low lumbar spine bone mineral density: A 2-year placebo-controlled randomized trial. Spine (Philos. Pa 1976) 24, 704–708 (1999).
Yang, J. H., Kim, J. H., Lim, D. S. & Oh, K. J. Effect of combined sex hormone replacement on bone/cartilage turnover in a murine model of osteoarthritis. Clin. Orthop. Surg. 4, 234–241 (2012).
Acknowledgements
The authors gratefully acknowledge the China Scholarship Council (CSC) scholarship to H.P. and the Australian Centre for HIV and Hepatitis Virology Research (ACH2) grant and University of Queensland Knowledge Exchange & Translation Fund to F.Y.H. The authors acknowledge the contribution by the University of Queensland Promoting Women Fellowship and the UQ Research Stimulus Fellowship to F.Y.H. This work is also supported by the Natural Science Foundation of China (No. 81871800 and 82072496) granted to W.D.; the Natural Science Foundation of Hebei Province (No. H2020206203) granted to S.Y.; and a National Health and Medical Research Council (NHMRC) Australia Ideas Grant (No. 2011398) granted to D.H.. This work is supported by the Assistant Secretary of Defense for Health Affairs endorsed by the US Department of Defense through the FY19 Chronic Pain Management Research Program (D.M.K: award no. W81XWH2010909).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pang, H., Chen, S., Klyne, D.M. et al. Low back pain and osteoarthritis pain: a perspective of estrogen. Bone Res 11, 42 (2023). https://doi.org/10.1038/s41413-023-00280-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41413-023-00280-x