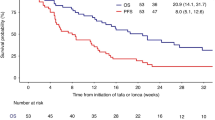
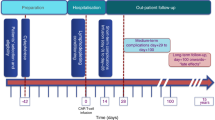
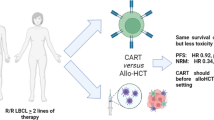
Abstract
In recent years, chimeric antigen receptor T-cell therapy (CAR T) has revolutionized the treatment landscape for large B cell lymphoma (LBCL), demonstrating remarkable efficacy and ushering a new era of therapeutic possibilities. However, a subset of patients may not achieve the desired response with CAR T. This review examines strategies aimed at optimizing outcomes for patients who relapse or progress after CAR T. Available data on utilization of CD19-directed monoclonal antibodies and antibody drug conjugates have shown limited efficacy in this setting. Moreover, bispecific antibodies have also emerged as an alternative therapy in relapsed and or refractory LBCL, but long-term follow up treated cases post-CAR T failure are lacking. Several observational studies have shown efficacy of allogeneic hematopoietic cell transplantation, but attainment of a complete remission prior to allografting is a prerequisite to achieve durable remissions. As we navigate the intricate landscape of treatment of post CAR T failure, it becomes evident that this represents a therapeutic challenge which necessitates a multifaceted approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Abramson, Palomba JS, Gordon LI ML, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Kanate AS, Majhail N, DeFilipp Z, Dhakal B, Dholaria B, Hamilton B, et al. Updated indications for immune effector cell therapy: 2023 guidelines from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2023;594:597–29.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26:1247–56.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
Mohty R, Moustafa MA, Aljurf M, Murthy H, Kharfan-Dabaja MA. Emerging role of autologous CD19 CAR T-cell therapies in the second-line setting for large B-cell lymphoma: a game changer? Hematol Oncol Stem Cell Ther. 2022;15:73–80.
Mohty R, Moreno Vanegas Y, Chavez JC, Kharfan-Dabaja MA. Lisocabtagene maraleucel for relapsed or refractory large B-cell non-Hodgkin lymphoma. Expert Rev Anticancer Ther. 2023;23:121–6.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5:4149–55.
Plaks V, Rossi JM, Chou J, Wang L, Poddar S, Han G, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138:1081–5.
Iqbal M, Bansal R, Yassine F, Gandhi S, Rosenthal A, Moustafa MA, et al. Impact of rituximab and corticosteroids on late cytopenias post-chimeric antigen receptor T cell therapy. Transpl Cell Ther. 2022;28:668.e1–e6.
Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21:978–88.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800.
Iqbal M, Khurana A, Chavez J, Rosenthal AC, Li Z, Craver E, et al. Efficacy of CD19 directed therapies in large B-cell lymphoma (LBCL) relapsing after chimeric antigen receptor (CAR) T-cell therapy. Blood. 2022;140:10396–8.
Qualls D, Buege MJ, Dao P, Caimi PF, Rutherford SC, Wehmeyer G, et al. Tafasitamab and lenalidomide in relapsed/refractory large B cell lymphoma (R/R LBCL): real world outcomes in a multicenter retrospective study. Blood. 2022;140:787–9.
Zurko JC, Epperla N, Nizamuddin I, Torka P, David KA, Ollila TA, et al. Outcomes and treatment patterns in patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy. Blood. 2021;138:884.
Nagler A, Perriello VM, Falini L, Falini B. How I treat refractory/relapsed diffuse large B-cell lymphomas with CD19-directed chimeric antigen receptor T cells. Br J Haematol. 2023;201:396–410.
Administration USFaD. FDA approves polatuzumab vedotin-piiq for previously untreated diffuse large B-cell lymphoma, not otherwise specified, and high-grade B-cell lymphoma. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-polatuzumab-vedotin-piiq-previously-untreated-diffuse-large-b-cell-lymphoma-not.
Administration USFaD. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-polatuzumab-vedotin-piiq-diffuse-large-b-cell-lymphoma.
Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar MK, Hess B, et al. A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Adv. 2022;6:2757–62.
Falchi L, Vardhana SA, Salles GA. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities. Blood. 2023;141:467–80.
Karimi Y, Ghesquieres H, Jurczak W, Cheah C, Clausen M, Lugtenburg P, et al. Effect of follow-up time on the ability of subcutaneous epcoritamab to induce deep and durable complete remissions in patients with relapsed/refractory large B-cell lymphoma: updated results from the pivotal EPCORE NHL-1 trial. J Clin Oncol. 2023;41:7525.
Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387:2220–31.
Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41:2238–47.
Dickinson M, Carlo-Stella C, Morschhauser F, Falchi L, Bachy E, Cartron G, et al. Glofitamab monotherapy in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL): extended follow-up and landmark analyses from a pivotal phase II study. Hematol Oncol. 2023;41:144–6.
Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022;40:481–91.
Administration USFaD. FDA D.I.S.C.O. Burst Edition: FDA approval of Lunsumio (mosunetuzumab-axgb) for adult patients with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-lunsumio-mosunetuzumab-axgb-adult-patients-relapsed-or#:~:text=On%20December%2022%2C%202022%2C%20the,more%20lines%20of%20systemic%20therapy.
Bartlett NL, Assouline S, Giri P, Schuster SJ, Cheah CY, Matasar M, et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2023;7:4926–35.
Olszewski AJ, Budde LE, Chavez J, Ghosh N, Kamdar M, Lossos IS, et al. Mosunetuzumab with polatuzumab vedotin is effective and has a manageable safety profile in patients aged <65 and ≥65 years with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) and ≥1 prior therapy: subgroup analysis of a phase Ib/II study. Blood 2022;140:3757–9.
Patel K, Michot J-M, Chanan-Khan A, Ghesquieres H, Bouabdallah K, Byrd JC, et al. Safety and anti-tumor activity of plamotamab (XmAb13676), an Anti-CD20 x Anti-CD3 bispecific antibody, in subjects with relapsed/refractory non-hodgkin’s lymphoma. Blood. 2021;138:2494.
Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, et al. Odronextamab, a human CD20xCD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327–e39.
Hou JZ, Jacobs R, Cho SG, Devata S, Gaballa S, Yoon DH, et al. Interim results of the phase 1 study of Tnb-486, a novel CD19xCD3 T-cell engager, in patients with relapsed/refractory (R/R) B-NHL. Blood. 2022;140:1474–5.
Shah NN, Hamadani M. Is there still a role for allogeneic transplantation in the management of lymphoma? J Clin Oncol. 2021;39:487–98.
Kharfan-Dabaja MA, El-Jurdi N, Ayala E, Kanate AS, Savani BN, Hamadani M. Is myeloablative dose intensity necessary in allogeneic hematopoietic cell transplantation for lymphomas? Bone Marrow Transpl. 2017;52:1487–94.
Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174:235–48.
Bacher U, Klyuchnikov E, Le-Rademacher J, Carreras J, Armand P, Bishop MR, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–62.
van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–8.
Sirvent A, Dhedin N, Michallet M, Mounier N, Faucher C, Yakoub-Agha I, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Biol Blood Marrow Transplant. 2010;16:78–85.
Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-Hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol. 2020;6:1011–8.
Epperla N, Ahn KW, Khanal M, Litovich C, Ahmed S, Ghosh N, et al. Impact of reduced-intensity conditioning regimens on outcomes in diffuse large B cell lymphoma undergoing allogeneic transplantation. Transpl Cell Ther. 2021;27:58–66.
Dreger P, Dietrich S, Schubert ML, Selberg L, Bondong A, Wegner M, et al. CAR T cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood Adv. 2020;4:6157–68.
Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2023;141:2307–15.
Dreger P, Fenske TS, Montoto S, Pasquini MC, Sureda A, Hamadani M. Cellular immunotherapy for refractory diffuse large b cell lymphoma in the chimeric antigen receptor-engineered T cell era: still a role for allogeneic transplantation? Biol Blood Marrow Transpl. 2020;26:e77–e85.
Zurko J, Ramdial J, Shadman M, Ahmed S, Szabo A, Iovino L, et al. Allogeneic transplant following CAR T-cell therapy for large B-cell lymphoma. Haematologica. 2023;108:98–109.
Fried S, Shouval R, Walji M, Flynn JR, Yerushalmi R, Shem-Tov N, et al. Allogeneic hematopoietic cell transplantation after chimeric antigen receptor T cell therapy in large B cell lymphoma. Transpl Cell Ther. 2023;29:99–107.
Shadman M, Gauthier J, Hay KA, Voutsinas JM, Milano F, Li A, et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy. Blood Adv. 2019;3:3062–9.
Administration USFaD. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma.
Locke FL, Bartlett NL, Jacobson CA, Oluwole OO, Munoz J, Lekakis LJ, et al. Retreatment (reTx) of patients (pts) with refractory large B-cell lymphoma with axicabtagene ciloleucel (axi-cel) in ZUMA-1. J Clin Oncol. 2020;38:8012.
Gauthier J, Bezerra ED, Hirayama AV, Fiorenza S, Sheih A, Chou CK, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137:323–35.
Vanegas YM, Mohty R, Gadd ME, Luo Y, Aljurf M, Qin H, et al. CAR-T cell therapies for B-cell lymphoid malignancies: identifying targets beyond CD19. Hematol Oncol Stem Cell Ther. 2022;15:81–93.
Baird JH, Frank MJ, Craig J, Patel S, Spiegel JY, Sahaf B, et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood. 2021;137:2321–5.
Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26:1569–75.
Locke FL, Miklos DB, Tees M, Bees T, Li A, Truppel-Hartmann A, et al. CRC-403: a phase 1/2 study of bbT369, a dual CD79a and CD20 targeting CAR T cell drug product with a gene edit, in relapsed and/or refractory B cell non-hodgkin’s lymphoma (NHL). Blood. 2022;140:12716–7.
Iacoboni G, Iraola-Truchuelo J, Mussetti A, Fernández-Caldas P, Navarro Garcés V, Martin Lopez AA, et al. Salvage treatment with novel agents is preferable to standard chemotherapy in patients with large B-cell lymphoma progressing after chimeric antigen receptor t-cell therapy. Blood. 2022;140:378–80.
Spiegel JY, Dahiya S, Jain MD, Tamaresis J, Nastoupil LJ, Jacobs MT, et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood 2021;137:1832–5.
Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, et al. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139:1026–38.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Song MK, Park BB, Uhm JE. Resistance mechanisms to CAR T-cell therapy and overcoming strategy in B-cell hematologic malignancies. Int J Mol Sci. 2019;20:5010
Erbella F, Bachy E, Cartron G, Gat E, Morschhauser F, Tessoulin B, et al. Late failure of aggressive B-cell lymphoma following CAR T-cell therapy: a lysa study from the descar-T registry. Blood. 2022;140:1325–7.
Dodero A, Bramanti S, Di Trani M, Pennisi M, Ljevar S, Chiappella A, et al. Outcome after chimeric antigen receptor (CAR) T-cell therapy failure in large B-cell lymphomas. Br J Haematol. 2023. Online ahead of print. https://doi.org/10.1111/bjh.19057
Author information
Authors and Affiliations
Contributions
SEW, MAKD, MI, MH, JC, and RM designed, wrote, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RM, SEW, and MI have no COI to declare; MAK-D: Research/grant: Novartis, Bristol Myers Squibb and Pharmacyclics. MH: Research Support/Funding: ADC Therapeutics; Spectrum Pharmaceuticals; Astellas Pharma. Consultancy: ADC Therapeutics, Omeros, CRISPR, BMS, Kite. Abbvie, Caribou, Genmab. Speaker’s Bureau: ADC Therapeutics, AstraZeneca, BeiGene, Kite. DMC: Inc, Genentech, Myeloid Therapeutics, CRISPR; JC: Consulting: BMS, Kite/Gilead, Novartis, GenMab, Genentech, AstraZeneca, Janssen, AdiCet, Cellectis, BeiGene, ADC Therapeutics. Honoraria: Lilly, AstraZeneca, BeiGene. Research Support: Merck, Janssen, AstraZeneca, Adaptive
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Warrak, S., Kharfan-Dabaja, M.A., Iqbal, M. et al. Therapeutic options for large B-cell lymphoma relapsing after CD19-directed CAR T-cell therapy. Bone Marrow Transplant 59, 162–170 (2024). https://doi.org/10.1038/s41409-023-02176-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02176-0