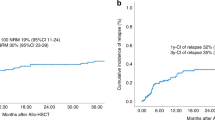
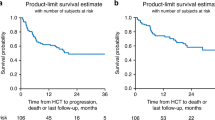
Abstract
Anti-CD19 chimeric antigen receptor T cells (CART) has rapidly been adopted as the standard third-line therapy to treat aggressive B-cell lymphomas (ABCL) after failure of second-line therapy despite the lack of direct comparisons with allogeneic hematopoietic cell transplantation (alloHCT)-based strategies. Using the Grupo Español de Trasplante y Terapia Celular (GETH-TC) registry, we selected patients with the following characteristics: CART or alloHCT performed between 2016 and 2021; ≥18 years old; ABCL diagnosis; ≥2 lines of therapy; and either anti-CD19 CART or alloHCT as therapy at relapse. The analysis included a total of 316 (CART = 215, alloHCT = 101) patients. Median follow-up was 15 and 36 months for the CART and alloHCT cohorts, respectively. In the multivariate analysis, CART was confirmed to be similar to alloHCT for the primary study endpoint (progression-free survival) (hazard ratio [HR] 0.92, CI95%:0.56–1.51, p = 0.75). Furthermore, when the analysis was limited to only patients with chemo-sensitive diseases (complete and partial response) at infusion (CART = 26, alloHCT=93), no differences were reported (progression-free survival at month +18: 65% versus 55%, p = 0.59). However, CART had lower non-relapse mortality (HR 0.34, 95% CI: 0.13–0.85, p = 0.02). Given the lower toxicity and similar survival outcomes, these results suggest the use of CART before alloHCT.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
19 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41409-023-01967-9
References
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. JCO. 2020;38:3119–28.
Bethge WA, Martus P, Schmitt M, Holtick U, Subklewe M, von Tresckow B, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022;140:349–58.
Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V, et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2022. https://doi.org/10.3324/haematol.2022.280805.
Bastos-Oreiro M, Gutierrez A, Reguera JL, Iacoboni G, López-Corral L, Terol MJ, et al. Best treatment option for patients with refractory aggressive B-cell lymphoma in the CAR-T cell era: real-world evidence from GELTAMO/GETH Spanish Groups. Front Immunol. 2022;13:855730.
Kuhnl A, Roddie C, Kirkwood AA, Tholouli E, Menne T, Patel A, et al. A national service for delivering CD19 CAR‐T in large B‐cell lymphoma—The UK real‐world experience. Br J Haematol. 2022;198:492–502.
Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174:235–48.
Bento L, Gutiérrez A, Novelli S, Montoro J, Piñana JL, López-Corral L, et al. Allogeneic stem cell transplantation as a curative option in relapse/refractory diffuse large B cell lymphoma: Spanish multicenter GETH/GELTAMO study. Bone Marrow Transpl. 2021;56:1919–28.
McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng G-S, et al. Survival, Nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020; 172:229.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and Non-Hodgkin lymphoma: the Lugano classification. JCO. 2014;32:3059–67.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti–T-lymphocyte globulin to assess impact on chronic graft-versus-host disease–free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. JCO. 2017;35:4003–11.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33:259–75.
Iacobelli S, On behalf of the EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2013;48:S1–S37.
Hu Z-H, Wang H-L, Gale RP, Zhang M-J. A SAS macro for estimating direct adjusted survival functions for time-to-event data with or without left truncation. Bone Marrow Transplant. 2022;57:6–10.
Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Prog Biomed. 2011;101:87–93.
R Core Team (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.
Sermer D, Batlevi C, Palomba ML, Shah G, Lin RJ, Perales M-A, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4:4669–78.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Dreger P, Dietrich S, Schubert M-L, Selberg L, Bondong A, Wegner M, et al. CAR T cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood Adv. 2020;4:6157–68.
Hamadani M, Gopal AK, Pasquini M, Kim S, Qiu X, Ahmed S, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6:486–94.
Sidana S, Dueck AC, Thanarajasingam G, Griffin JM, Thompson C, Durani U, et al. Longitudinal patient reported outcomes with CAR-T cell therapy versus autologous and allogeneic stem cell transplant. Transplant Cell Ther. 2022;28:473–82.
Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
Acknowledgements
We thank CERCA Programme/Generalitat de Catalunya for institutional support. We thank Ángel Cedillo for his contribution in obtaining clinical data for the manuscript.
Author information
Authors and Affiliations
Contributions
AM, LB, MBO were responsible for the overall research questions and design of the study. AM, BRS performed the statistical analyses. AM, LB, MBO wrote the original draft. CBMD and SA contributed equally as senior authors. All authors reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
We declare no other competing interests for the execution of this study. The data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.21772991.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The author’s name Ana Jiménez-Ubieto has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mussetti, A., Bento, L., Bastos-Oreiro, M. et al. Role of allogeneic hematopoietic cell transplant for relapsed/refractory aggressive B-cell lymphomas in the CART era. Bone Marrow Transplant 58, 673–679 (2023). https://doi.org/10.1038/s41409-023-01949-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01949-x
This article is cited by
-
Outcomes of allogeneic hematopoietic stem cell transplantation for relapsed or refractory diffuse large B-cell lymphoma
Bone Marrow Transplantation (2024)
-
Allogeneic stem cell transplantation and CAR-T in B-cell Non-Hodgkin Lymphoma: a two-center experience and review of the literature
Annals of Hematology (2024)