Abstract
We retrospectively compared outcomes of 404 MDS patients undergoing 1st matched sibling donor allo-HCT receiving either PTCy-based (n = 66) or other “conventional prophylaxis” (n = 338; mostly calcineurin inhibitor + methotrexate or MMF). Baseline characteristics were balanced, except for higher use of myeloablative regimens in the PTCy group (52.3% vs. 38.2%, p = 0.047). Incidences of neutrophil (Day +28: 89% vs. 97%, p = 0.011) and platelet (Day +100: 89% vs. 97%, p < 0.001) engraftment were lower for PTCy-based. Day +100 cumulative incidences of grade II–IV and III–IV aGVHD, and 5-year CI of extensive cGVHD were 32%, 18% and 18% for PTCy-based and 25% (p = 0.3), 13% (p = 0.4) and 31% (p = 0.09) for the conventional cohort. Five-year OS (51% vs. 52%, p = 0.6) and GRFS (33% vs. 25%, p = 0.6) were similar between groups. Patients receiving PTCy had a trend to a lower cumulative incidence of relapse (20% vs. 33%, p = 0.06), not confirmed on multivariable analysis (p = 0.3). Although higher NRM rates were observed in patients receiving PTCy (32% vs. 21%, p = 0.02) on univariate analysis, this was not confirmed on multivariate analysis (HR 1.46, p = 0.18), and there was no resultant effect on OS (HR 1.20, p = 0.5). Based on these data, PTCy prophylaxis appears to be an attractive option for patients with MDS undergoing MSD allo-HCT.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) remains the only potentially curative treatment option for patients with myelodysplastic syndrome (MDS) [1]. Registry data from the EBMT confirms increasing MDS allo-HCT activity, with >2500 registered transplants per annum in recent years [2]. However, long-term success is challenged by the risks of relapse, graft-versus-host disease (GVHD), and non-relapse mortality (NRM). GVHD remains a major cause of transplant-related morbidity and can significantly impact quality of life. The Chronic Malignancies Working Party of the EBMT previously evaluated the impact of T cell depletion (TCD) strategies with either single agent anti-thymocyte globulin (ATG) or alemtuzumab in a large cohort of MDS patients who underwent allo-HCT [3]. Both agents decreased the risk of chronic GVHD however on multivariable analyses, the use of ATG was associated with improved GVHD-free relapse-free survival (GRFS), whereas alemtuzumab was associated with an increased risk of relapse and worse overall survival (OS).
Following the successful use of post-transplant cyclophosphamide (PTCy) in the haploidentical donor (HID) setting, first demonstrated over two decades ago, utilization of PTCy for GVHD prevention, either alone or in combination with agents such as ATG, is becoming increasingly prevalent across other transplant platforms [4,5,6]. PTCy-based prophylaxis had been extended to matched sibling donor (MSD) allo-HCT with notable success [7,8,9,10]. However, despite these encouraging results with regards to GVHD prevention, wider implementation has additionally highlighted potential risks such as delayed engraftment, increased infective episodes, and specific organ toxicity, such as early cardiac complications in some cases, although this remains under debate [11,12,13,14].
Limited studies to date have focused on the efficacy and safety of using PTCy-based prophylaxis compared to conventional approaches in patients with MDS undergoing MSD allo-HCT [4]. Considering that NRM after allo-HCT in patients with MDS remains significantly relevant and that PTCY-based prophylaxis has been associated with post-transplant complications derived from the profound immunosuppression derived from this approach and specific organ toxicity, the present analysis compares the outcomes of PTCy-based versus conventional “other” GVHD prophylaxis strategies in a contemporaneous cohort of MDS patients undergoing MSD allo-HCT from the EBMT registry.
Methods
This was a retrospective, multicenter, registry-based analysis approved by the Chronic Malignancies Working Party of the EBMT. The EBMT is a non-profit, scientific society representing more than 600 transplant centers mainly in Europe. Data are entered, managed, and maintained in a central database with internet access; each EBMT center is represented in this database. The patient selection included patients undergoing first allo-HCT for MDS between 2014 and 2020, using either reduced-intensity conditioning (RIC) or myeloablative conditioning (MAC) as defined by standard EBMT criteria, receiving either PTCy-based or other conventional GvHD prophylaxis [15]. Allo-HCT conducted using other donor types, alternative stem cell sources, or ex vivo T-cell depletion were excluded. Performance status was assessed via the reported Karnofsky Performance Status (KPS) and comorbidities via the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) [16].
Based on these criteria, an initial 1268 adults were identified in the EBMT Registry database but missing data in some compromised inclusion. All related variables present in the EBMT registry were extracted in June 2022. Thereafter, all the relevant EBMT center members were invited to participate in the study data initiative—follow-up questionnaires (MED-C forms) were generated for centers to improve data completeness and returned between January 2023 and June 2023. A total of 52 institutions agreed to participate leading to a total study cohort of 404 patients for whom missing information considered relevant for the conduction of the study was requested and received from participating centers between January 2023 and June 2023.
Neutrophil engraftment was defined as the time at which the absolute neutrophil count was >0.5 × 109/L for 3 consecutive days and platelet engraftment as a platelet count >20 × 109/L for 7 consecutive days without transfusion support. Primary graft failure (PGF) was defined as failing to reach neutrophil >0.5 × 109/L in the first 28 days post stem cell transplantation or documentation of autologous reconstitution by chimerism analysis in the absence of relapse [17]. Secondary graft failure was defined by the treating physician: standard criteria across Europe would be loss of a functioning graft demonstrated by cytopenia in at least two lineages and loss of donor chimerism. CR (complete remission) was defined if all the following were achieved: Hb >11 g/dL, Platelet >100 × 109/L and Neutrophils >1.5 × 109/L with less than 5% blast in the bone marrow. Relapse was defined as loss of CR. For this study CR and relapse were designated by the treating physician. Conditioning regimes were defined as myeloablative conditioning (MAC) if they contained either total body irradiation (TBI) with a dose of >6 Gy, oral Busulfan dosage >8 mg/kg or a dose of intravenous Busulfan >6.4 mg/kg [15]. Grading of acute GVHD (aGVHD) and chronic GVHD (cGVHD) was performed using established criteria [18,19,20]. Following the information reported in the EBMT registry, the severity of cGVHD was graded according to the classic criteria (limited vs. extensive). Chronic GVHD was considered limited if it is present only in the liver and/or a localized area of the skin. If the cGvHD affected any other organ(s) or there was generalized skin involvement, it was considered to be extensive. The study was approved by the CMWP of the EBMT institutional review board and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Statistical analysis
The type of GVHD prophylaxis used for allo-HCT (PTCY-based vs. other prophylaxis) was considered the main variable of interest in this study. The primary outcomes were OS, progression-free survival (PFS), grade III–IV aGvHD and extensive cGvHD-free and relapse-free survival (GRFS), relapse, NRM, and aGvHD and cGvHD. Secondary outcomes were neutrophil engraftment, platelet engraftment, cardiac toxicity, and pulmonary toxicity.
OS, PFS, and GRFS were estimated using the Kaplan–Meier product limit estimation method, and differences in subgroups were assessed by the Log-Rank test. Median follow-up was determined using the reverse Kaplan–Meier method. The cumulative incidences of relapse/NRM, aGvHD II–IV and III–IV, overall cGvHD and limited and extensive cGvHD were analyzed separately in a competing risks framework. In all GvHD-related outcomes, relapse and death were considered competing events. Relapse and death were competing events for NRM and relapse incidence, respectively. Competing risk analyses were also used to analyze the cumulative incidences of neutrophil engraftment, platelet engraftment, cardiac toxicity and pulmonary toxicity separately, each with competing event death. Subgroup differences in cumulative incidences were assessed using Gray’s test. In univariable analyses, patients alive and in follow-up by 5 years after transplant were censored at that time, with the exception of aGvHD, platelet and neutrophil engraftment, which were censored at days 100, 100 and 28, respectively.
The impact of the main explanatory variable (type of GVHD prophylaxis), and other risk factors, in OS, PFS, NRM and CIR were explored using multivariable Cox regression analyses. The baseline risk factors included in each of the multivariable models were selected based on clinical judgment prior to the analysis. Missing values were excluded except in TP53, where the missing indicator method was applied. All models included the main study variable GvHD prophylaxis (PTCy-based, other + ATG vs other - ATG). Any other included covariates are considered adjustment factors. The models of OS and PFS additionally included the covariate constellation Age at allo-HCT (by decade), IPSS-R at diagnosis (High, Very High vs Low/Intermediate), TP53 mutation (Present, Missing vs Absent), Year of allo-HCT (by year) and Conditioning (RIC vs MAC). Cause-specific hazard models were fitted for relapse and NRM. The model of relapse included the covariates Age at allo-HCT (by decade), HCT-CI (High vs Low/Intermediate), Karnofsky score (<80 vs 90–100) and Conditioning (RIC vs MAC). The model of NRM included TP53 mutation (Present, Missing vs Absent), IPSS-R at diagnosis (High, Very High vs Low/Intermediate), Disease status (CR, untreated vs active disease) and Conditioning (RIC vs MAC). P values are provided by unadjusted Wald tests.
Continuous pre-transplant variables were summarized by the median and interquartile range (IQR) and categorical pre-transplant variables are summarized as percentages within the group of patients with available data. Group differences between the PTCy-based and other prophylaxis subgroups were assessed by X2-tests for categorical baseline variables and by t-tests for continuous baseline variables. All p values were two-sided and p < 0.05 was considered significant. Statistical analyses were performed in R version 3.6.0 (R Development Core Team, Vienna, Austria), using packages “survival”, “prodlim”, “cmprsk” and “risk Regression”.
Results
Baseline patient, disease and transplant characteristics
A total of 404 MDS patients undergoing their first MSD allo-HCT using PB-derived stem cells between 2014 and 2020 from 52 participating centers, receiving either PTCy-based (n = 66) or other “conventional prophylaxis” (n = 338) for GVHD, were included. Patient characteristics, disease risk, and pre-transplant disease status were mostly balanced between the two cohorts as shown in Table 1. The median age of those in the PTCy-based and “conventional prophylaxis” cohort was 54 (interquartile range (IQR), 41–64) and 58 (IQR, 51–63) years, respectively (p = 0.06). A majority of patients in both cohorts were male; “PTCy-based” (n = 37 (56.1%)) and “conventional prophylaxis” (n = 238 (70.4%)). At the time of diagnosis, all patients met established criteria for MDS and according to the revised International Prognostic Scoring System (IPSS-R, Table 1), the majority of patients in both cohorts had intermediate, high- or very high-risk disease. Where screening had been performed, a TP53 mutation was positive in 26 (21.1%) of the 123 processed samples from the entire cohort but was not enriched in either prophylaxis group. Thirteen (19.7%) patients in the PTCy-based cohort and 72 (21.3%) in the other “conventional prophylaxis” cohort had transformed to AML prior to allo-HCT. Overall, the proportions of patients who underwent treatment prior to allo-HCT were similar between the two study groups (n = 45 (71.4%) in PTCy-based and n = 230 (70.3%) in conventional prophylaxis, p = 0.98)). The proportion of patients in the PTCy-based and conventional prophylaxis cohorts with a KPS < 90% was 27.3% vs. 34.4% (p = 0.3) and with an HCT-CI > 3 were 31.8% vs. 35.3%, (p = 0.8), respectively.
Patients were most frequently transplanted within the first year from diagnosis, with a median time to the transplant of 6.4 (IQR, 3.3–15.3) months for “PTCy-based” versus 7 (IQR, 4.2–13.8) months for the “conventional prophylaxis” group, respectively. The majority of transplant characteristics were balanced between the two study groups, except for the proportion of patients who received MAC, which was higher in the PTCy group (52.3% vs. 38.2%, p = 0.047).
For the PTCy-based cohort, PTCy was combined with at least two additional immunosuppressant agents in 66.7% of the cases, with one additional immunosuppressive drug (generally calcineurin inhibitors (CNI)) in 31.8% of the cohort and administered as a single agent in only one (1.5%) case. ATG was administered in 6.1% (n = 4) of cases. The other “conventional prophylaxis” cohort mainly combined CNI with either mycophenolate mofetil (MMF; n = 155) or methotrexate (MTX; n = 183), and in 162 (47.9%) cases, ATG was also administered. Median follow-up (IQR) after transplantation was 3.8 (IQR, 3.3–4.5) and 4.7 (IQR,4.2–5.1) years for the “PTCy-based” and “conventional prophylaxis” groups, respectively.
Engraftment and GVHD
Neutrophil engraftment by day +28 was documented for 59 (89%) and 325 (97%) of the PTCy-based and conventional prophylaxis cohorts, respectively (p = 0.011). However, the median of days to neutrophil engraftment; estimated in patients who did engraft in the first 28 days, was similar between the two study groups (18 (IQR 14–21) vs. 16 (IQR 13–19) days, p = 0.064). The day +100 incidence of platelet engraftment was lower in the PTCY-based cohort (89% vs. 97%, p < 0.001), and occurring in a median of 21 days (IQR 16–27.8), compared with 14 (12–17) days (p < 0.001) in the non-PTCy group.
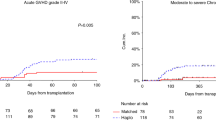
As shown in Fig. 1, the day +100 cumulative incidences of grade II–IV and III–IV aGVHD, and 5-year CI of cGVHD (any grade) and extensive cGVHD were 32% (95% CI 21–43), 18% (95% CI 9–27), 44% (95% CI 32–57) and 18% (95% CI 9–28) for patients who received PTCy-based prophylaxis and 25% (95% CI 20–30) (p = 0.3), 13% (95% CI 10–17) (p = 0.4), 48% (95% CI 42–53) (p = 0.7) and 31% (95% CI 26–36) (p = 0.09) for those who did not.
Post-transplant complications: infections and organ toxicity
CMV and EBV reactivation occurred in 37/65 (56%) and 1/50 (2%) of patients who received PTCy-based 106/338 (31%) and 57/338 (17%) for those who received conventional prophylaxis. The day 100 cumulative incidence of fungal infections in the PTCy-based and conventional prophylaxis cohorts was 10% (95% CI 2–17%) and 7% (95% CI 4–10%) respectively (p = 0.4). The cumulative incidence of post-transplant lymphoproliferative disorders was similar between the two groups (1% (95% CI 0–2) by five in conventional prophylaxis and not observed in PTCy).
Specific organ toxicity was additionally investigated. As reported in Table 2, The day +100 cumulative incidence of cardiac toxicity was higher in the PTCY-based cohort (13% (95% CI 1–25) vs. 8% (95% CI 4–12), p = 0.6), with no statistically significant differences in between. However, the cumulative incidence of cardiac toxicity diagnosed during the first 5 years after allo-HCT was comparable between the two study groups (5 years: 23% (95% CI 6–40) vs. 19% (95% CI 12–25), p = 0.6). Of note, pulmonary toxicities (5 years: 23% (95% CI 8–38) vs. 27% (95% CI 21–34), p = 0.6) did not differ between the PTCy-based and conventional prophylaxis cohorts.
Overall survival, relapse-free survival and GVHD-free relapse-free survival
On univariate analysis, there were no significant differences between the two groups with respect to OS, PFS, and GRFS (Fig. 2 and Table 2). The estimated 5-year OS, PFS, and GRFS of the patients who received PTCy-based prophylaxis were 51% (95% CI 39–64), 48% (95% CI 36–61), and 33% (95% CI 21–45), and for the patients who received conventional GVHD prophylaxis were 52 (95% CI 46–58), 46% (95% CI 40–52), and 25 (95% CI 20–30), respectively. Results observed in the univariate analysis were confirmed in the multivariable regression analyses (MVA) adjusted by variables considered clinically relevant. As reported in Table 3, using PTCy-based prophylaxis resulted in comparable OS (HR 1.20 (95% CI 0.73–1.99), p = 0.5) PFS (HR 1.15 (95% CI 0.71–1.85), p = 0.6) and GRFS (HR 0.82 (95% CI 0.55–1.24), p = 0.3) to conventional prophylaxis without ATG. Furthermore, regardless of the GVHD prophylaxis approach, the diagnosis of high or very high-risk MDS (according to IPSS-R), and the presence of TP53 mutation at diagnosis (where that information was available) were independent predictors of worse post-transplant outcomes.
Relapse incidence and non-relapse mortality
During the first 5 years post allo-HCT, disease relapse was documented in 119 (29%) of the entire cohort, at a median of 6.2 (2.9–12) months after allo-HCT. As shown in Table 2 and Fig. 2, patients who received PTCy-based prophylaxis had a lower CIR (20% (95% CI 10–29) vs. 33% (95% CI 28–38), p = 0.06), but higher NRM rates (32% (95% CI 20–43) vs. 21% (95% CI 16–25), p = 0.09). Results provided from the MVA confirmed that the use of PTCy-based prophylaxis resulted in analogous incidences of disease relapse compared to the use of conventional prophylaxis (HR 0.83 (95% CI 0.42–1.66), p = 0.6) (Table 4). Furthermore, regardless of the immunosuppressive agents used for GVHD prevention, patients classified into the very-high-risk group (HR 2.06 (95% CI 1.20–3.55), p = 0.009) according to the IPSS-R and the presence of a TP53 mutation (HR 1.88 (95% CI 0.93–3.81), p = 0.08) at diagnosis were found to be predictive of disease relapse after allo-HCT.
Based on the results provided by the univariate analysis, the effect of the GVHD prophylaxis in NRM was explored in detail. The MVA reported in Table 4 demonstrated that, compared with allo-HCT performed from other prophylaxis without ATG, using PTCy-based prophylaxis associated comparable NRM (HR 1.46 (95% CI 0.85–2.50), p = 0.18), and a non-significant trend to lower NRM was observed in allo-HCT performed from ATG-based prophylaxis (HR 0.61 (95% CI 0.36–1.02), p = 0.06). In addition, patients with a higher number of comorbidities (HCT-CI > 2) had an increased risk for NRM than the rest (HR 1.80 (95% CI 1.16–2.8), p = 0.009). Older age, worse KPS, and the administration of MAC regimens were not associated with higher transplant-related mortality on MVA.
Causes of death
During the 5 years post allo-HCT, 185 (45%) of the patients in the entire cohort died, and amongst them, 31 (17%) had received PTCy. Primary causes of death differed according to the GVHD prophylaxis strategy (p < 0.006). For instance, disease recurrence accounted for death in 10% of patients receiving PTCy and 31.0% of those who did not. Of particular note, GVHD and infectious complications accounted for death in 26.7% and 46.7% of patients included in the PTCy-based cohort and 38.1% and 22.8% for those included in the conventional prophylaxis cohort.
Discussion
Many questions remain concerning the best GVHD prophylactic strategy across donor types and transplant platforms for MDS allo-HCT. In the present study, we highlight that the use of PTCy-based prophylaxis in adults undergoing MSD first allo-HCT for MDS using PB-derived stem cells results in comparable OS, RFS, and GRFS rates to conventional GVHD prophylaxis strategies. Patients receiving PTCy had a non-significant trend to lower incidence of relapse, but a higher NRM than the rest observed only in the univariate analysis. Furthermore, allo-HCT performed with PTCy, was associated with a lower incidence of neutrophil and platelet engraftment at day +28 and +100, respectively, and comparable incidences of clinically relevant aGVHD. Of note, utilization of PTCy was associated with a non-significant trend to a lower incidence of extensive cGVHD in univariate analysis; this is of note as 48% of patients in the “conventional prophylaxis” cohort received ATG. Nevertheless, the results obtained in NRM question whether PTCY should be extensively used in patients with MDS undergoing allo-HCT from PB MSD grafts, especially in patients who present additional risk factors for NRM, such as an HCT-CI > 2.
Since the advent of PTCy-based GVHD prophylaxis in HID allo-HCT, demonstrating low rates of aGVHD/cGVHD, increasing studies have highlighted utility across a vast range of disease types/ risk, donor types, and transplant platforms [5, 21]. Brissot et al. on behalf of the acute leukemia working party (ALWP) of the EBMT evaluated PTCy versus ATG in AML patients in CR1 undergoing 10/10 MUD allo-HCT in a retrospective, EBMT registry-based study [22]. Here, PTCy and ATG had comparable incidences of aGVHD grade II–IV, cGVHD and extensive cGVHD. No differences were evident in OS, RFS, GRFS or NRM, also confirmed in a matched-pair analysis. Battipaglia, also on behalf of the ALWP, alternatively reported on PTCy versus ATG in the MSD setting for AML in CR1. Here, although patients were younger in the PTCy group with concomitant higher use of MAC regimens, there was no difference in OS, leukemia-free survival, or GRFS [9]. Matched-pair analysis confirmed a lower cumulative incidence of cGVHD with ATG, which was also upheld when only PBSC allo-HCT was considered. Mehta et al. from MD Anderson reported on a retrospective evaluation of 964 patients who received Tacrolimus (TAC)/MTX ± ATG (n = 578) versus PTCy-based (n = 386) GVHD prophylaxis [23]. For the MSD cohort, 140 patients received PTCy-based prophylaxis and 272 patients received TAC/MTX. For MUD, 246 received PTCy and 306 TAC/MTX/ATG. The majority underwent MAC and had PB as a stem cell source. Both in the MSD and MUD setting, PTCy was associated with higher incidences of delayed neutrophil engraftment, bacterial infection and hemorrhagic cystitis. MVA revealed similar rates of aGVHD grade III–IV for both cohorts and a significantly lower risk of cGVHD in the MSD setting and improved GRFS for both MUD and MSD following PTCy-based prophylaxis. More recently, Bolaños-Meade et al. compared, in a prospective randomized study, the efficacy of PTCY/TAC/MMF (n = 214) with TAC/MTX (n = 217) in RIC allo-HCT performed from MSD, MUD, and 9/10 MMUD [24]. PTCY-based prophylaxis was associated with higher GRFS secondary to less severe acute and chronic GVHD. PTCY/TAC/MMF was associated with a higher incidence of immunosuppression-free survival at 1 year, and with comparable overall and disease-free survival, relapse and NRM. In our study, regarding engraftment, PTCy-based prophylaxis was associated with a lower incidence of day +28 neutrophil engraftment, delayed platelet recovery, and a 3% incidence of primary GF. Dosing strategies of standard PTCy dose (50 mg/kg/day) and timing (days +3 and +4) may be associated with both delayed engraftment and early post-allo-HCT toxicity. Delayed haematopoietic recovery has been previously explored and may reflect direct stem cell toxicity which relates to dosing and timing of the Cy, the use of bone marrow with PTCy and the use of agents in addition to PTCy as GVHD prophylaxis. The early study by Kanakry et al. with a small number of PTCy-only prophylaxed BM grafts resulted in equivalent engraftment when compared to MSD allografts with alternative GVHD prophylaxis [5]. In attempting to understand this, Marco-Ayala et al. reviewed transfusion requirements following PTCy-based (n = 100) versus other “CNI-based” prophylaxis (n = 100) in a retrospective study for MSD allo-HCT, showing delayed neutrophil engraftment paralleled with higher red blood cell and platelet transfusion requirements during the first-month post-allo-HCT for the PTCy group [11]. Increasing consideration is hence being given to the dosing and timing of PTCy. McAdams et al. have reported on a single center phase I/II trial of de-escalating PTCy dosing in fludarabine and busulfan-conditioned haploidentical allo-HCT (BM stem cell source), demonstrating that de-escalating to 25 mg/kg/day PTCy (day+3 and +4) was feasible and associated with more rapid engraftment compared to standard dosing [25]. Such studies and the need for additional immunosuppressants are required in the MSD and MUD setting to assess if improvements in engraftment kinetics without an abrogation of the benefits of PTCy-based prophylaxis can be achieved.
Regarding GVHD, similar incidences of aGVHD II–IV were seen in both the PTCy and conventional cohorts, which is reassuring although conclusions are limited by the heterogeneous use of ATG across cohorts and the fact that we do not have robust detailing on ATG dosing/ schedule which may clearly significantly influence clinical outcomes. Despite almost half of the patients in the conventional cohort getting ATG, a non-significant trend to lower extensive cGVHD by 5 years was noted in the PTCy cohort on univariable analysis suggesting, alongside the aGVHD data, that PTCy-based prophylaxis in this setting moderates GVHD rates at least as appropriately as conventional prophylaxis. Reassuringly, the acceptable GVHD risks associated with PTCy were not associated with higher rates of relapse, as patients receiving PTCy had comparable relapse rates than those who did not. As per previous studies, we show that both higher IPSS-R classification (very high risk versus “other”) and the presence of a TP53 mutation are associated with a higher risk of relapse, including in the PTCy prophylaxis cohort [26, 27].
Of interest when considering the cause of death in both cohorts, despite no differences in OS, infection accounted for more deaths in the PTCy cohort than relapse and GVHD combined, whereas in the conventional prophylaxis cohort, the leading causes were GVHD and relapse. We showed a differential viral reactivation with a lower incidence of EBV reactivation (2% vs 17%) in the PTCY cohort, whereas CMV reactivation was higher in the PTCY group (56% vs 31%) but there was no difference in the incidence of reported fungal infections. As this is a registry-based study, we do not have data on bacterial infections. Previous studies have reported on delayed T cell subpopulation kinetics following the use of PTCy. Khimani et al. reported across a range of HID and MUD allo-HCT that absolute CD4+ recovery was delayed in the PTCy-based cohorts compared to conventional prophylaxis and there was a significantly higher incidence of infectious complications within the first year, including higher rates of CMV and BK virus reactivation but with a similar 2-year OS and NRM [28].
A significantly higher NRM rate in the PTCy arm was observed only in univariate analysis, with infection as opposed to GVHD being a dominant cause of NRM. This result may relate to delayed neutrophil engraftment and increased rates of primary and secondary graft failure. In addition, the higher proportion of patients transplanted using MAC regimens in the PTCy group may have indirectly contributed to the higher NRM rates observed in the univariate analysis reported in Fig. 2. Since the intensity of the conditioning regimen is generally tailored according to chronological age and patient fitness, the differences observed in conditioning intensity would be related to the differences on median age (chronological age), KPS, and HCT-CI trends observed in patients included in each cohort. However, since the present study is retrospective and multicenter, the policy of deciding whether patients received MAC or RIC conditionings may vary between centers. Lastly, the higher trend of cardiac toxicity diagnosed during the first 100 days after the stem cell infusion may have also contributed to the increased NRM observed in the PTCy arm [14, 29].
Lastly, it is important to remark that when the effect of PTCy-based prophylaxis in NRM was explored in detail, the results were not confirmed in the multivariable model, suggesting that PTCY-based prophylaxis results in comparable rates of NRM when compared with allo-HCT performed from other prophylaxis without ATG, and that the use of ATG-based prophylaxis might be beneficial in a certain group of patients transplanted from MSD for MDS. Additional studies should be conducted to investigate the role of ATG-based prophylaxis in MSD allo-HCT for MDS, as the present one has been designed to better address the utility of PTCy-based prophylaxis in this setting. Hence, and despite the negative association observed between using PTCY and increased NRM in the univariate model, using PTCy resulted in comparable post-transplant outcomes to other prophylaxis among these patients.
Limitations of our study are those inherent to large registry-based studies such as the retrospective, registry-based nature, reasoning for physician choice for allocation to differing GVHD prophylaxis strategies, heterogeneous use of ATG and potential center effects on dosing and the fact that data on immunosuppressant tapering were not provided. Furthermore, the fact that ATG was more often used in RIC allo-HCT limited the distribution of patients included in the non-PTCY group in two independent groups of patients to additionally compare the results of ATG-containing prophylaxis with those obtained from using PTCy.
Lastly, and despite the entire EBMT center members were invited to participate, the relatively low number of patients who were finally included in the study is considered a limitation of the power of the statistical analysis. Nonetheless, to date, this remains the largest study evaluating PTCy-based approaches in MSD PBSC allo-HCT for MDS compared to conventional prophylaxis. Data highlights acceptable GVHD rates, with a trend to less extensive cGVHD at 5 years, and similar OS and RFS rates. NRM rates and infectious complications remain to be addressed considering the “added value” of PTCy prophylaxis in this setting. Prospective controlled studies with uniform PTCy/ATG dosing and conditioning for MDS allo-HCT would be warranted to directly answer the questions raised by this analysis.
Data availability
Data sharing would only be considered for research purposes after specific requests and internal revision and consideration.
References
Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28:2235–40.
Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Cámara R, Dolstra H, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transplant. 2023;58:647–58.
Forcade E, Chevret S, Finke J, Ehninger G, Ayuk F, Beelen D, et al. Impact of in vivo T-cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: a registry study from the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2022;57:768–74.
Alanazi W, Chen S, Lipton JH, Kim DD, Viswabandya A, Kumar R, et al. Post-transplant cyclophosphamide combined with anti-thymocyte globulin as graft-versus-host disease prophylaxis for allogeneic hematopoietic cell transplantation in high-risk acute myeloid leukemia and myelodysplastic syndrome. Acta Haematol. 2021;144:66–73.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA-matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Bailén R, Pascual-Cascón MJ, Guerreiro M, López-Corral L, Chinea A, Bermúdez A, et al. Post-transplantation cyclophosphamide after HLA identical compared to haploidentical donor transplant in acute myeloid leukemia: a study on behalf of GETH-TC. Transplant Cell Ther. 2022;28:204.e1–10.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43.
Battipaglia G, Labopin M, Hamladji R, Blaise D, Chevallier P, Brissot E, et al. Post‐transplantation cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation from HLA‐identical sibling donors: a retrospective analysis from the acute leukemia working party of the European society for blood and marrow transplantation. Cancer. 2021;127:209–18.
Lazzari L, Balaguer-Roselló A, Montoro J, Greco R, Hernani R, Lupo-Stanghellini MT, et al. Post-transplant cyclophosphamide and sirolimus based graft-versus-host disease prophylaxis after allogeneic stem cell transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2022;57:1389–98.
Marco-Ayala J, Sanz J, Gómez-Seguí I, Balaguer-Rosello A, Montoro J, Guerreiro M, et al. Impact of post-transplantation cyclophosphamide on transfusion requirements in HLA-matched sibling peripheral blood stem cell transplantation. Transplant Cell Ther. 2023;29:313.e1–10.
Lin C, Vader JM, Slade M, DiPersio JF, Westervelt P, Romee R. Cardiomyopathy in patients after posttransplant cyclophosphamide–based hematopoietic cell transplantation. Cancer. 2017;123:1800–9.
Yeh J, Whited L, Saliba RM, Rondon G, Banchs J, Shpall E, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5:5599–607.
Duléry R, Mohty R, Labopin M, Sestili S, Malard F, Brissot E, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol. 2021;3:250–9.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Carreras E, Dufour C, Kröger N, Mohty M, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham: Springer International Publishing; 2019. https://doi.org/10.1007/978-3-030-02278-5.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from Hl-A-matched sibling donors. Transplantation. 1974;18:295–304.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Risk-adapted GVHD prophylaxis with post-transplantation cyclophosphamide in adults after related, unrelated, and haploidentical transplantations. Eur J Haematol. 2018;100:395–402.
Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13:87.
Mehta RS, Saliba RM, Rondon G, Al-Atrash G, Bashir Q, Hosing CM, et al. Post-transplantation cyclophosphamide versus tacrolimus and methotrexate graft-versus-host disease prophylaxis for HLA-matched donor transplantation. Transpl Cell Ther. 2022;28:695.e1–10.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48.
McAdams MJ, Hyder M, Dimitrova D, Sadler JL, McKeown C, Steinberg SM, et al. Phase I/II study of reduced dosing of post-transplantation cyclophosphamide (PTCy) after HLA-haploidentical bone marrow transplantation. Blood. 2021;138:101.
Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376:536–47.
Della Porta MG, Alessandrino EP, Bacigalupo A, van Lint MT, Malcovati L, Pascutto C, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123:2333–42.
Khimani F, Ranspach P, Elmariah H, Kim J, Whiting J, Nishihori T, et al. Increased infections and delayed CD4+ T cell but faster B cell immune reconstitution after post-transplantation cyclophosphamide compared to conventional GVHD prophylaxis in allogeneic transplantation. Transplant Cell Ther. 2021;27:940–8.
Pérez-Valencia AI, Cascos E, Carbonell-Ordeig S, Charry P, Gómez-Hernando M, Rodríguez-Lobato LG, et al. Incidence, risk factors, and impact of early cardiac toxicity after allogeneic hematopoietic cell transplant. Blood Adv. 2023;7:2018–31.
Author information
Authors and Affiliations
Contributions
DM, MR, RK, DJE, and MQS designed the study. DJE did the statistical analysis. DM, DJE, and MQS interpreted the results and wrote the manuscript. MR and RF provided valuable support for interpreting the results and statement of the conclusions. LK, JM, JP, FF, AECB, JK, NK, ZNO, MJPC, UP, GVG, TM, TLLP, MM, SS, MK, FO, CG, CS, and JDS LGRL provided valuable input into the study and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salas, M.Q., Eikema, DJ., Koster, L. et al. Impact of post-transplant cyclophosphamide (PTCy)-based prophylaxis in matched sibling donor allogeneic haematopoietic cell transplantation for patients with myelodysplastic syndrome: a retrospective study on behalf of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 59, 479–488 (2024). https://doi.org/10.1038/s41409-023-02159-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02159-1