Abstract
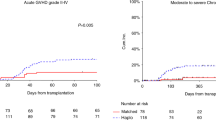
We retrospectively compared outcomes of 404 MDS patients undergoing 1st matched sibling donor allo-HCT receiving either PTCy-based (n = 66) or other “conventional prophylaxis” (n = 338; mostly calcineurin inhibitor + methotrexate or MMF). Baseline characteristics were balanced, except for higher use of myeloablative regimens in the PTCy group (52.3% vs. 38.2%, p = 0.047). Incidences of neutrophil (Day +28: 89% vs. 97%, p = 0.011) and platelet (Day +100: 89% vs. 97%, p < 0.001) engraftment were lower for PTCy-based. Day +100 cumulative incidences of grade II–IV and III–IV aGVHD, and 5-year CI of extensive cGVHD were 32%, 18% and 18% for PTCy-based and 25% (p = 0.3), 13% (p = 0.4) and 31% (p = 0.09) for the conventional cohort. Five-year OS (51% vs. 52%, p = 0.6) and GRFS (33% vs. 25%, p = 0.6) were similar between groups. Patients receiving PTCy had a trend to a lower cumulative incidence of relapse (20% vs. 33%, p = 0.06), not confirmed on multivariable analysis (p = 0.3). Although higher NRM rates were observed in patients receiving PTCy (32% vs. 21%, p = 0.02) on univariate analysis, this was not confirmed on multivariate analysis (HR 1.46, p = 0.18), and there was no resultant effect on OS (HR 1.20, p = 0.5). Based on these data, PTCy prophylaxis appears to be an attractive option for patients with MDS undergoing MSD allo-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing would only be considered for research purposes after specific requests and internal revision and consideration.
References
Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28:2235–40.
Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Cámara R, Dolstra H, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transplant. 2023;58:647–58.
Forcade E, Chevret S, Finke J, Ehninger G, Ayuk F, Beelen D, et al. Impact of in vivo T-cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: a registry study from the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2022;57:768–74.
Alanazi W, Chen S, Lipton JH, Kim DD, Viswabandya A, Kumar R, et al. Post-transplant cyclophosphamide combined with anti-thymocyte globulin as graft-versus-host disease prophylaxis for allogeneic hematopoietic cell transplantation in high-risk acute myeloid leukemia and myelodysplastic syndrome. Acta Haematol. 2021;144:66–73.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA-matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Bailén R, Pascual-Cascón MJ, Guerreiro M, López-Corral L, Chinea A, Bermúdez A, et al. Post-transplantation cyclophosphamide after HLA identical compared to haploidentical donor transplant in acute myeloid leukemia: a study on behalf of GETH-TC. Transplant Cell Ther. 2022;28:204.e1–10.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43.
Battipaglia G, Labopin M, Hamladji R, Blaise D, Chevallier P, Brissot E, et al. Post‐transplantation cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation from HLA‐identical sibling donors: a retrospective analysis from the acute leukemia working party of the European society for blood and marrow transplantation. Cancer. 2021;127:209–18.
Lazzari L, Balaguer-Roselló A, Montoro J, Greco R, Hernani R, Lupo-Stanghellini MT, et al. Post-transplant cyclophosphamide and sirolimus based graft-versus-host disease prophylaxis after allogeneic stem cell transplantation for acute myeloid leukemia. Bone Marrow Transplant. 2022;57:1389–98.
Marco-Ayala J, Sanz J, Gómez-Seguí I, Balaguer-Rosello A, Montoro J, Guerreiro M, et al. Impact of post-transplantation cyclophosphamide on transfusion requirements in HLA-matched sibling peripheral blood stem cell transplantation. Transplant Cell Ther. 2023;29:313.e1–10.
Lin C, Vader JM, Slade M, DiPersio JF, Westervelt P, Romee R. Cardiomyopathy in patients after posttransplant cyclophosphamide–based hematopoietic cell transplantation. Cancer. 2017;123:1800–9.
Yeh J, Whited L, Saliba RM, Rondon G, Banchs J, Shpall E, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5:5599–607.
Duléry R, Mohty R, Labopin M, Sestili S, Malard F, Brissot E, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol. 2021;3:250–9.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Carreras E, Dufour C, Kröger N, Mohty M, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham: Springer International Publishing; 2019. https://doi.org/10.1007/978-3-030-02278-5.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from Hl-A-matched sibling donors. Transplantation. 1974;18:295–304.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Risk-adapted GVHD prophylaxis with post-transplantation cyclophosphamide in adults after related, unrelated, and haploidentical transplantations. Eur J Haematol. 2018;100:395–402.
Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13:87.
Mehta RS, Saliba RM, Rondon G, Al-Atrash G, Bashir Q, Hosing CM, et al. Post-transplantation cyclophosphamide versus tacrolimus and methotrexate graft-versus-host disease prophylaxis for HLA-matched donor transplantation. Transpl Cell Ther. 2022;28:695.e1–10.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48.
McAdams MJ, Hyder M, Dimitrova D, Sadler JL, McKeown C, Steinberg SM, et al. Phase I/II study of reduced dosing of post-transplantation cyclophosphamide (PTCy) after HLA-haploidentical bone marrow transplantation. Blood. 2021;138:101.
Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376:536–47.
Della Porta MG, Alessandrino EP, Bacigalupo A, van Lint MT, Malcovati L, Pascutto C, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123:2333–42.
Khimani F, Ranspach P, Elmariah H, Kim J, Whiting J, Nishihori T, et al. Increased infections and delayed CD4+ T cell but faster B cell immune reconstitution after post-transplantation cyclophosphamide compared to conventional GVHD prophylaxis in allogeneic transplantation. Transplant Cell Ther. 2021;27:940–8.
Pérez-Valencia AI, Cascos E, Carbonell-Ordeig S, Charry P, Gómez-Hernando M, Rodríguez-Lobato LG, et al. Incidence, risk factors, and impact of early cardiac toxicity after allogeneic hematopoietic cell transplant. Blood Adv. 2023;7:2018–31.
Author information
Authors and Affiliations
Contributions
DM, MR, RK, DJE, and MQS designed the study. DJE did the statistical analysis. DM, DJE, and MQS interpreted the results and wrote the manuscript. MR and RF provided valuable support for interpreting the results and statement of the conclusions. LK, JM, JP, FF, AECB, JK, NK, ZNO, MJPC, UP, GVG, TM, TLLP, MM, SS, MK, FO, CG, CS, and JDS LGRL provided valuable input into the study and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salas, M.Q., Eikema, DJ., Koster, L. et al. Impact of post-transplant cyclophosphamide (PTCy)-based prophylaxis in matched sibling donor allogeneic haematopoietic cell transplantation for patients with myelodysplastic syndrome: a retrospective study on behalf of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 59, 479–488 (2024). https://doi.org/10.1038/s41409-023-02159-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02159-1