Abstract
Hematopoietic cell transplantation from haploidentical donors (haploHCT) has facilitated treatment of AML and MDS by increasing donor availability and became more feasible since the introduction of post-transplant cyclophosphamide (ptCY). In our single-center retrospective analysis including 213 patients with AML or MDS, we compare the outcome of haploHCT (n = 40) with ptCY with HCT from HLA-identical MRD (n = 105) and MUD (n = 68). At 2 years after transplantation, overall survival (OS) after haploHCT was not significantly different (0.59; 95% confidence interval 0.44–0.79) compared to MRD (0.77; 0.67–0.88) and MUD transplantation (0.72; 0.64–0.82, p = 0.51). While progression-free survival (PFS) was also not significantly different (haploHCT: 0.60; 0.46–0.78, MRD: 0.55; 0.44–0.69, MUD: 0.64; 0.55–0.74, p = 0.64), non-relapse mortality (NRM) was significantly higher after haploHCT (0.18; 0.08–0.33) vs. MRD (0.029; 0.005–0.09) and MUD (0.06; 0.02–0.12, p < 0.05). Higher NRM was mainly caused by a higher rate of fatal infections, while deaths related to GvHD or other non-relapse reasons were rare in all groups. As most fatal infections occurred early and were bacterial related, one potential risk factor among many was identified in the significantly longer time to neutrophil engraftment after haploHCT with a median of 16 days (interquartile range; 14.8–20.0) vs. 12 days (10.0–13.0) for MRD and 11 days (10.0–13.0) for MUD (p = 0.01).
Similar content being viewed by others
Introduction
The introduction of allogeneic hematopoietic cell transplantation (HCT) from related, human leukocyte antigen (HLA) haploidentical donors (haploHCT) led to an increased availability of potential donors and thereby facilitated treatment of acute myeloid leukemia (AML) and myelodysplastic neoplasm (MDS) [1]. Previously avoided due to a higher risk of fatal infections and graft-versus-host disease (GvHD) [2,3,4,5], haploHCT has become a valid and widely used treatment option when combined with post-transplant cyclophosphamide (ptCY) [6, 7]. More recently, ptCy has also been shown to improve outcome for matched donor transplantation [8]. While initially mainly performed with bone marrow, subsequent studies demonstrated comparable results with mobilized peripheral blood stem cells (PBSC) [9]. Immunosuppression with ptCY together with mycophenolate mofetil (MMF) and a calcineurin inhibitor (CNI; ciclosporine A (CSA) or tacrolimus) minimizes the risk for acute and chronic GvHD and non-relapse mortality (NRM) early post-transplant by depletion of early alloreactive T cells [6, 10]. This is explained by their higher susceptibility to cyclophosphamide compared to resting T cells due to their cycling activity and proliferation, before they expand and infiltrate GvHD target organs [11]. Nevertheless, retrospective analyses in AML suggest that overall survival (OS) after haploHCT with ptCY still tends to be inferior compared with HCT from standard HLA- matched (min. 10/10) unrelated (MUD) or related (sibling) donors (MRD). However, published survival data are mostly from retrospective analyses and do not consistently reveal whether this trend is explained by a higher relapse rate or higher non-relapse mortality (NRM) [10, 12,13,14]. As a recent example, Mehta and colleagues highlighted that although haploHCT is associated with similar rate of relapse and severe GvHD, higher NRM was observed and primarily attributed to higher rates of fatal infections due to delayed T-cell reconstitution compared with MRD or MUD-HCTs [14,15,16]. To minimize infection risk, broad antimicrobial prophylaxis protocols have been implemented, generally combining antibiotics, antifungals, and antivirals, while substances vary substantially among different centers [15].
Here we report our single-center experience with haploHCT performed with ptCY, MMF and CNI as GvHD prophylaxis and compare it with MUD- and MRD-HCT performed with conventional immunosuppression. We demonstrate differences in NRM and cause of death distribution between groups, but not in OS and PFS and hypothesize on possible reasons for reported differences.
Methods
Patients
We retrospectively analyzed all patients over the age of 18 years diagnosed with AML or MDS who underwent first HCT between January 1st 2015 and December 31st 2020 at the Zurich University Hospital’s Department of Medical Oncology and Hematology in Zurich, Switzerland. We excluded transplantations from cord blood, unrelated HLA-mismatched donors, and manipulated grafts (such as ex-vivo CD34+ enrichment). We categorized patients according to donor type; related haploidentical, MRD and MUD matched at HLA-A*, B*, C*, DQB1* and DRB1* (minimum 10 of 10 HLA match), respectively. Original data was extracted continuously from electronic medical records by trained personnel and was later revised and analyzed by the authors.
Endpoints
The primary objective of the study was to compare OS between groups. Secondary endpoints were PFS, NRM as well as causes of death and time from HCT to engraftment of neutrophils and platelets.
Furthermore, we documented prevalence of severe acute GvHD (aGvHD, grade II or higher) and peripheral whole blood chimerism on day 100 posttransplant. aGvHD was generally assessed and graded according to the consortium criteria published by Harris et al. [17].
Cause of death was categorized as either disease progression, GvHD, infection, or others (including graft failure (GF) and sinusoidal obstruction syndrome of the liver (SOS)). Since infections accompanied most deaths, death from infectious complications was only classified when there was no evidence of severe GvHD or morphological relapse. When both progression and severe GvHD and “other reasons” applied, progression was prioritized. Infectious deaths were further classified by the microorganism primarily responsible for the fatal outcome (bacterial, fungal, viral).
Time to engraftment of neutrophils (>1 × 10^9/L) and platelets (>50 × 10^9/L) was defined as time from HCT to the first day off at least three consecutive days above threshold without transfusions.
End of follow-up was June 30st 2021 representing at least 6 months follow-up after the last patient’s transplantation.
Conditioning regimens
Patients who underwent haploHCT received either reduced intensity conditioning (RIC) with fludarabine (30 mg/m2 body surface, d-7 to −2) and busulfan (4 × 1 mg/kg body weight, d-3 & −2, concentration of steady state (CSS) adapted) or myeloablative conditioning (MAC) with busulfan (4 × 1 mg/kg d-7 to −4, CSS adapted) and cyclophosphamide (50 mg/kg, d-3 & d-2).
5 patients received an alternative RIC regimen with FluCy-sTBI (fludarabine 30 mg/m2, d-6 to −2; cyclophosphamide 14.5 mg/kg, d-6 and −5, and single-dose total-body-irradiation, 2 Gy on d-1). Immunosuppression after haploHCT included CSA (until d + 180), MMF (dose reduction from day +35) and ptCY (50 mg/kg, d + 3 & +4) according to published protocols [6].
Patient with MUD or MRD transplantation received either RIC with fludarabine, busulfan (dosage as above), and anti-thymocyte-globulin (ATG Neovii©, 10 mg/kg/d on days −4 to −1) or MAC with busulfan, cyclophosphamide, and ATG (s. above). G-CSF support was administered in all patients until neutrophil recovery >1 × 10^9/L. Immunosuppression (IS) in MRD and MUD-HCT was performed with CSA (dose reduction from day +100) and MMF (dose reduction from day+28 in MRD-HCT, d + 56 in MUD-HCT) after RIC, and CSA and methotrexate (MTX) after MAC (MTX 15 mg/kg bodyweight on d + 1, 10 mg/kg on d + 3, d + 6 and d + 11).
Transplant and infection-related definitions
Antimicrobial prophylaxis was started after transplantation and always included valacyclovir and pneumocystis prophylaxis (usually trimethoprim-sulfamethoxazole) for at least 6 months. After haploHCT, antifungal prophylaxis with an azole (usually posaconazole) and antibiotics (usually levofloxacin) were added until IS cessation. Later, after its approval in Switzerland in 2020, cytomegaly-virus (CMV) prophylaxis with letermovir was introduced in high-risk HCT recipients.
In case of a suspected infection, empiric broad-spectrum antibiotic treatment with pseudomonas coverage was generally initiated (usually 4th generation cephalosporine or piperacillin/tazobactam). If imaging studies showed evidence of systemic fungal disease, antifungal prophylaxis was changed to another therapy, usually amphotericin B i.v. or another azole p.o. in the outpatient setting.
Valacyclovir at a prophylactic dose (500 mg bid) was dose escalated in case of signs of symptomatic herpes simplex or herpes zoster disease. CMV replication in the blood was monitored weekly by serum PCR, and treatment with valganciclovir was initiated in case of CMV disease or if copy numbers repeatedly reached >1000 IU/ml.
Statistical analysis
Data were analyzed using R Studio [18] version 4.0.5. Comparisons between groups were analyzed using the Wilcoxon rank sum test (continuous data), Pearson’s chi-square test, or Fisher’s exact test (categorical data) as appropriate to test for statistical significance. Continuous data were summarized as median and interquartile range (IQR). Categorical data were summarized as numbers (n) and frequency (%). Figures were created using version 3.3.5 of ggplot2 [19].
OS and PFS was estimated using the Kaplan-Meier method, log-rank test was used to evaluate differences between groups (packages survival and survminer [20], version 0.4.9). Surviving patients were censored at the date of last follow-up. PFS was defined as time from HCT to death from any cause or progression. Accordingly, patients who died before experiencing relapse were defined to have a competing event.
NRM was defined as death without relapse with relapse classified as a competing event. Probabilities of NRM were estimated with the use of cumulative incidence curves, Gray’s method was used to evaluate differences [21]. Missing data were dealt with by excluding patients from particular analyses if their file did not contain data for the required variables.
Hazard ratios for OS and PFS were evaluated using Cox proportional hazards regression analysis and Gray competing-risk regression analysis for NRM.
The cumulative incidence method was used to calculate incidence of causes of death over time from transplantation [21]. Here, no censoring for competing risks was performed. All p-values were adjusted (Bonferroni correction), p < 0.05 was considered statistically significant.
Ethics approval
All patients provided informed consent according to the local institutions practice. The local ethics committee granted ethical approval for the study (BASEC No. 2022–00861) in accordance with the principles of the Declaration of Helsinki and its amendments [22].
Results
Patient characterisics
A total of 213 patients were included in this analysis, all underwent HCT as part of treatment for AML or MDS. 40 had a haploidentical donor, 105 a MUD and 68 a MRD.
Baseline characteristics are shown in Table 1. In all three cohorts, patients’ characteristics regarding age at diagnosis and transplantation, distribution of gender and ethnicity, Karnofsky performance status at transplantation, CD34 + PBSC count, and CMV risk status were similar. The haploHCT group had fewer patients with MDS and more with AML, fewer patients received myeloablative conditioning (MAC) and more were in morphologic complete remission (CR) at transplantation, although molecular remission rates and the distribution of ELN2022 risk groups at diagnosis were similar in all groups. As expected, donors in the MUD group were younger and more often male. The median duration of follow-up was 617 days (20.5 months, range 6.2–78.0 months).
Survival, PFS and NRM
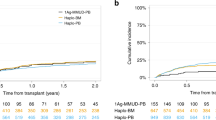
Differences in OS grew over time from transplantation but did not reach statistical significance. At 2 years after HCT, probability of OS after haploHCT was 0.5 (95% confidence interval; 0.44–0.79) compared to 0.77 (0.67–0.88) after MRD and 0.72 (0.64–0.82) after MUD transplantation (Fig. 1a, Log-rank; p = 0.51). In the univariate analysis, hazard ratio for OS at 2 years was 0.54 for MRD (0.26–1.14) and 0.62 for MRD (0.32–1.19) compared to haploHCT.
PFS also was not significantly different between groups. At 24 months, probability of PFS after haploHCT was 0.60 (95% CI; 0.46–0.78), 0.55 (0.44, 0.69) after MRD-HCT, and 0.64 (0.55–0.74) after MUD-HCT (Fig. 1b, Log-rank; p = 0.64). HR for PFS at 2 years was 1.08 (0.58–2.03) for MRD and 0.87 (0.48–1.59) for MUD compared to haploHCT.
However, NRM was significantly higher after haploHCT with a cumulative incidence at 2 years of 0.18 (95% CI, 0.08–0.33) versus 0.029 (0.005–0.09) after MRD-HCT and 0.06 (0.02–0.12) after MUD-HCT (Fig. 1c, p = 0.01). HR for NRM at 2 years was 0.31 (0.10–0.92) for MRD and 0.31 (0.03–0.80) for MUD compared to haploHCT.
Causes of death
Cumulative incidence of causes of death demonstrate higher NRM after haploHCT is mainly caused by a higher rate of fatal infections (Fig. 2b), with most infectious deaths occurring within the first 100 days after transplantation. At 2 years, cumulative incidence of death due to infection reaches 0.13 (95% CI; 0.046–0.259) after haploHCT versus 0.014 (0.0012–0.070) after MRD and 0.023 (0.0042–0.075) after MUD transplantation. Meanwhile, cumulative incidence of deaths due to relapse reaches 0.22 (95% CI; 0.09–0.38) of haploHCT, 0.21 (0.12–0.33) of MRD and 0.21 (0.13–0.30) of MUD-HCT. Accordingly, overall cause of death distribution at 2 years after transplant (Fig. 2a) shows a higher fraction of fatal infections after haploHCT (35.7%) compared to MRD (5.6%) and MUD transplantation (6.2%). The fraction of death due to relapse was lower after haploHCT (50%) than after MRD (88.9%) and MUD HCT (81.2%). Fatal, both acute or chronic GvHD and other reasons of death (including GF or SOS) were responsible for less than 10% of all deaths in all groups (GvHD; 7.1% of deaths after haploHCT vs. 5.6% after MRD-HCT and 6.2% after MUD-HCT, other reasons; 7.1% of deaths after haploHCT vs. 0% after MRD-HCT and 6.2% after MUD-HCT). Of Note, fatal SOS occurred only in 2 patients overall, both after MUD-HCT.
In the HaploHCT group, 4 out of 5 infectious deaths (n = 4/5) were caused by bacterial infections and 1 by uncontrolled fungal disease (n = 1/5). 3/5 deaths occurred before and 2/5 after neutrophil engraftment. In the MUD group (n = 2/2), both fatal infections were caused by bacterial sepsis after engraftment whereas after MRD transplantation (n = 1/1), the only fatal infection was due to a viral infection after neutrophil engraftment (cmv pneumonitis).
Engraftment, chimerism and GvHD
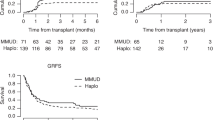
Time to engraftment of neutrophils was significantly longer after haploHCT with a median of 16.0 days (interquartile range (IQR); 14.8–20.0) versus 12.0 days after MRD (IQR; 10.0–13.0) and 11.0 days after MUD-HCT (IQR; 10.0–13.0) as demonstrate in Table 2 and Fig. 3 (p < 0.001). Accordingly, median time to engraftment of platelets after haploHCT was 26.5 days (IQR; 21.2–36.8) versus 16.0 days (13.0–21.0) after MRD and 15.0 days (12.0–19.0) after MUD-HCT (p < 0.001).
Follow-up Table 2 shows outcomes at day +100 and +730 (2 years) of follow-up. Until day +100, all cohorts reached a median 100% peripheral whole blood donor chimerism. At that time, cumulative incidence of acute GvHD grade II or higher was not statistically different after MRD-HCT (14.7%) compared to MUD-HCT (21.9%) and haploidentical HCT (27.5%, p = 0.2).
Discussion
The results of our single-center retrospective analysis including 213 patients with AML and MDS show no significant differences in OS and PFS but significantly higher NRM after haploHCT performed with ptCY compared to HCT from MRD and MUD performed with conventional immunosuppression. We demonstrate higher NRM is driven predominantly by a higher cumulative incidence of fatal infections, with most fatal infections occurring early after transplantation. Meanwhile, rates of death due to progression or other transplant-related causes, such as fatal GvHD, were comparably low in all groups. Our findings are important because they provide real-life confirmation for AML/MDS of what has been shown in reference studies [10, 12, 14] and therefore support the efficacy of this widely adopted regimen for haploidentical HCT. Our results appear to be representative since median OS and cumulative incidence of causes of death among groups are similar to those recently published by Mehta and colleagues [14]. However, unlike us, they demonstrate significant differences in OS between haploHCT and matched donor transplantation in an overall larger cohort.
Although other analyses have not come to the same conclusions [10, 12, 23], higher rates of NRM and fatal infections after haploHCT have been demonstrated in several studies [13, 14] and its reasons and mechanisms are a widely discussed topic.
One important factor may be impaired infection control due to slower donor-derived CD4+ T-cell reconstitution after haploHCT. Compared to ptCY-based transplantation from MRD, McCurdy et al. demonstrated slower recovery of CD4 + T-cells early post-transplant but not of CD8+, B- or NK-cells after haploHCT [24]. Furthermore, in our population, ptCY was administred only after haploHCT resulting in further T-cell suppression [14, 25,26,27].
Second, as supported by our analysis, another major reason may be the longer time to neutrophil engraftment. Although only incompletely understood, delayed engraftment is thought to lead to an increased risk of early bacterial and fungal infections [14, 23, 28]. Notably, delayed engraftment was observed in our analysis even though the proportion of PBSC grafts was high in all groups, suggesting that graft source is not the major cause for slower engraftment, as hypothesized by others [10, 23, 29]. Furthermore, although MUDs were slightly younger than haploidentical donors, we also cannot clearly associate delayed engraftment with higher donor age, since similar age differences between MRD and MUD did not result in the same trend. This is relevant because several studies have suggested an adverse outcome after transplantation from older unrelated donors [30,31,32,33].
Third, as a higher rate of GvHD has been historically attributed to HLA-mismatched transplantations [2, 3, 34], the reluctance to decrease systemic immunosuppression post-transplant may have led to delayed weaning in our cohort, thereby promoting infectious complications. Among possible implications of our findings, we hypothesize that a less intensive immunosuppression protocol after haploHCT could lead to an even better outcome through lower NRM. Although previously suggested, until today this concept has only been tested rigorously in matched donor transplantation [35,36,37,38]. Since the rate of fatal GvHD was low in all groups of our analysis, the study of earlier IS cessation could be justified also after haploHCT.
Furthermore, antimicrobial prophylaxis regimens could be modified to more effectively prevent infections, most importantly early post-transplant. However, as no universally applicable infection control protocols have been published to date and regimens vary considerably between centers worldwide, there are no universally accepted recommendations due to lack of comparability [15]. These efforts are further complicated by the different microbial resistance profiles in different parts of the world.
We conclude that haploHCT is safe and effective in the treatment of AML and MDS with similar rates of OS and PFS. The higher NRM is caused mainly by a higher incidence of fatal infections which could be favored by the longer time to neutrophil engraftment after haploHCT.
The strengths of our analysis, although not as comprehensive as many registry-based analyses, lie in the completeness of the data and the homogeneity of the transplant protocols and patient groups with its focus on AML/MDS, as well as the long observation period. We demonstrate a very similar distribution of disease risk between the groups according to the only recently published ELN2022 risk classification [39]. In contrast to reference studies [10, 12, 14, 23], the majority of patients we compared underwent transplantation with PBSC grafts, which allowed us to minimise a potential confounder of outcome measures.
Limitations
Apart from the above and the fact that this is a single-center, retrospective analysis, this study has several other limitations. Importantly, non-lethal infections were not systematically recorded. Within groups, different conditioning regimens were used and baseline characteristics of included patients vary slightly between groups. Furthermore, no systematic GvHD assessment after day +100 was documented, making it impossible to report GvHD-free survival as others have. In addition, fewer patients with MDS were included in the haploHCT group possibly altering its outcome, presumably because more time was available to find a MUD in these patients.
Data availability
All data can be made available upon reasonable request to the corresponding author.
References
Slade M, Fakhri B, Savani BN, Romee R. Halfway there: the past, present and future of haploidentical transplantation [Internet]. Bone Marrow Transpl. 2017;52:1–6. https://pubmed.ncbi.nlm.nih.gov/27454072/.
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol [Internet]. 1997;15:1767–77. https://pubmed.ncbi.nlm.nih.gov/9164184/.
Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol [Internet]. 1990;29:79–91. https://pubmed.ncbi.nlm.nih.gov/2249952/.
Koh LP, Chao N Haploidentical hematopoietic cell transplantation [Internet]. Bone Marrow Transplant. Nature Publishing Group; 2008 [cited 2022 Dec 16]. S60–3. Available from: https://www.nature.com/articles/bmt2008117.
Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States [Internet]. Bone Marrow Transpl. 2008;41:473–81. https://www.nature.com/articles/1705966.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl [Internet]. 2008;14:641–50. https://pubmed.ncbi.nlm.nih.gov/18489989/.
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transpl [Internet]. 2021;56:1651–64. https://doi.org/10.1038/s41409-021-01227-8.
Holtan SG, Hamadani M, WUJ AL, Malki MM, Runaas L, Elmariah H, et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for graft-versus-host disease (GVHD) prophylaxis in reduced intensity conditioning: results from phase III BMT CTN 1703. Blood [Internet]. 2022;140:LBA-4-LBA-4. https://ashpublications.org/blood/article/140/Supplement2/LBA-4/493428/Post-Transplant-Cyclophosphamide-Tacrolimus-and.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transpl [Internet]. 2012;18:1859–66. https://pubmed.ncbi.nlm.nih.gov/22863841/.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood [Internet]. 2015;126:1033–40. https://pubmed.ncbi.nlm.nih.gov/26130705/.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol [Internet]. 2012;39:683–93. https://pubmed.ncbi.nlm.nih.gov/23206845/.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol [Internet]. 2013;31:1310–6. https://pubmed.ncbi.nlm.nih.gov/23423745/.
Gooptu M, Romee R, St. Martin A, Arora M, Al Malki M, Antin JH, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood [Internet]. 2021;138:273–82. https://pubmed.ncbi.nlm.nih.gov/34292325/.
Mehta RS, Saliba RM, Ghanem S, Alousi AM, Rondon G, Anderlini P, et al. Haploidentical versus matched unrelated versus matched sibling donor hematopoietic cell transplantation with post-transplantation cyclophosphamide. Transpl Cell Ther [Internet]. 2022;28:395.e1–395.e11. https://linkinghub.elsevier.com/retrieve/pii/S2666636722012659.
Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis [Internet]. 2017;19:e12629. https://onlinelibrary.wiley.com/doi/10.1111/tid.12629.
Fayard A, Daguenet E, Blaise D, Chevallier P, Labussière H, Berceanu A, et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transpl [Internet]. 2019;54:1586–94. https://pubmed.ncbi.nlm.nih.gov/30770870/.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transpl [Internet]. 2016;22:4–10. https://pubmed.ncbi.nlm.nih.gov/26386318/.
RStudio Team. RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/. 2020;2019. Available from: https://www.rstudio.com/.
Wickham H. g gplot2: Elegant Graphics for Data Analysis [Internet]. 2nd ed. Springer; 2016. https://doi.org/10.1007/978-3-319-24277-4_5.
Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls [Internet]. Lancet. 2002;359:1686–9. https://pubmed.ncbi.nlm.nih.gov/12020548/.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med [Internet]. 1999;18:695–706. https://pubmed.ncbi.nlm.nih.gov/10204198/.
World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects [Internet]. JAMA - J. Am. Med. Assoc. American Medical Association; 2013 [cited 2022 Mar 18]. p. 2191–4. Available from: https://pubmed.ncbi.nlm.nih.gov/24141714/.
Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transpl [Internet]. 2014;20:1975–81. https://pubmed.ncbi.nlm.nih.gov/25263628/.
McCurdy SR, Luznik L. Immune reconstitution after T-cell replete HLA-haploidentical transplantation. Semin Hematol. 2019;56:221–6.
Russo A, Oliveira G, Berglund S, Greco R, Gambacorta V, Cieri N, et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: dynamics and clinical implications. Blood [Internet]. 2018;131:247–62. https://pubmed.ncbi.nlm.nih.gov/28986344/.
Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood [Internet]. 2015;125:2855–64. https://pubmed.ncbi.nlm.nih.gov/25742699/.
Fuchs EJ, McCurdy SR, Solomon SR, Wang T, Herr MR, Modi D, et al. HLA informs risk predictions after haploidentical stem cell transplantation with posttransplantation cyclophosphamide. Blood [Internet]. 2022;139:1452–68. https://ashpublications.org/blood/article/139/10/1452/477735/HLA-informs-risk-predictions-after-haploidentical.
Singh A, Dandoy CE, Chen M, Kim S, Mulroney CM, Kharfan-Dabaja MA, et al. Post-transplantation cyclophosphamide is associated with an increase in non-cytomegalovirus herpesvirus infections in patients with acute leukemia and myelodysplastic syndrome. Transpl Cell Ther. 2022;28:48.e1–48.e10.
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of Bone Marrow as Compared with Peripheral-Blood Cells from HLA-Identical Relatives in Patients with Hematologic Cancers. N. Engl J Med [Internet]. 2001;344:175–81. http://www.nejm.org/doi/abs/10.1056/NEJM200101183440303.
Boettcher S, Wilk CM, Singer J, Beier F, Burcklen E, Beisel C, et al. Clonal hematopoiesis in donors and long-term survivors of related allogeneic hematopoietic stem cell transplantation. Blood [Internet]. 2020;135:1548–59. https://pubmed.ncbi.nlm.nih.gov/32181816/.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl J Med [Internet]. 2014;371:2488–98. http://www.nejm.org/doi/10.1056/NEJMoa1408617.
Gibson CJ, Kim HT, Zhao L, Murdock HM, Hambley B, Ogata A, et al. Donor clonal hematopoiesis and recipient outcomes after transplantation. J Clin Oncol [Internet]. 2022;40:189–201. https://ascopubs.org/doi/10.1200/JCO.21.02286.
Xhaard A, Cunha R, Busson M, Robin M, Dhedin N, Coman T, et al. Clinical profile, biological markers, and comorbidity index as predictors of transplant-related mortality after allo-HSCT. Blood Adv [Internet]. 2017;1:1409–13. http://ashpublications.org/bloodadvances/article-pdf/1/18/1409/878434/advances008094.pdf.
Copelan EA. Hematopoietic stem-cell transplantation. N. Engl J Med [Internet]. 2006;354:1813–26. http://www.nejm.org/doi/abs/10.1056/NEJMra052638.
Kanakry CG, O’Donnell PV, Furlong T, De Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol [Internet]. 2014;32:3497–505. https://pubmed.ncbi.nlm.nih.gov/25267759/.
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol [Internet]. 2015;33:3152–61. https://pubmed.ncbi.nlm.nih.gov/26261255/.
Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot M, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: the prospective randomized HOVON-96 trial. Blood Adv [Internet]. 2022;6:3378–85. https://pubmed.ncbi.nlm.nih.gov/35143644/.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol [Internet]. 2022;40:356–68. https://pubmed.ncbi.nlm.nih.gov/34855460/.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood [Internet]. 2022;140:1345–77. http://ashpublications.org/blood/article-pdf/140/12/1345/1921355/bloodbld2022016867.pdf.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Contributions
Conceptualization: MR and SS. Data curation, investigation, and methodology: MR, SS. Funding acquisition: NA. Drafting of the original manuscript, project administration: MR, SS. Revision and Editing: DS, AM, RS, GN, US, MGM. Statistical analysis and visualization: SS and MR. Statistical software: R Studio Software (2020). Administrative, technical, and material support: MR, SS. Supervision and validation: US.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The local ethics committee (“Kantonale Ethikkomission Zurich”) granted ethical approval for the study (BASEC No. 2022–00861) in accordance with the principles of the Declaration of Helsinki [22].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rieger, M.J., Stolz, S.M., Müller, A.M. et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched related and unrelated donor transplant in acute myeloid leukemia and myelodysplastic neoplasm. Bone Marrow Transplant 58, 1121–1129 (2023). https://doi.org/10.1038/s41409-023-02042-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02042-z