Abstract
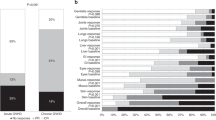
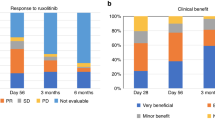
Imatinib is used for patients with SR-cGVHD. However, in 50% of cases imatinib is discontinued due to intolerance or inefficacy. In order to investigate nilotinib’s role as salvage therapy in those patients, we conducted a prospective, multicenter, phase II study. (NCT02891395). Patients with SR-cGVHD were included to receive imatinib. Patients who stopped imatinib due to intolerance or inefficacy switched to Nilotinib. The primary endpoint was defined as the week-12 response rate to Nilotinib. The response was considered successful if superior to the 30% endpoint. Sixty-two patients started the IM-phase. Fourteen patients (22%) discontinued imatinib before week 12 due to: cGVHD progression (10%) or TKI-class-specific intolerance (12%). At week 12, we observed complete remission in 13 patients (21%) and partial response in 8 patients (13%). Twenty-nine patients switched to Nilotinib. Nilotinib response at week-12 was observed in 6 patients (21%) while 23 patients (79%) discontinued Nilotinib due to intolerance/cGVHD progression. The primary endpoint was not reached. This prospective study confirmed the efficacy of imatinib in patients with steroid refractory cGVHD. It failed to demonstrate the efficacy of nilotinib as a salvage therapy in patients who were intolerant/unresponsive to imatinib.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. Bone Marrow Transpl. 2016;51:786–92.
Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation – A Report from CIBMTR. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:266–74.
Arora M, Klein JP, Weisdorf DJ, Hassebroek A, Flowers MED, Cutler CS, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood 2011;117:6714–20.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. N Engl J Med. 2012;367:https://doi.org/10.1056/NEJMoa1203517.
Ringdén O, Labopin M, Bacigalupo A, Arcese W, Schaefer UW, Willemze R, et al. Transplantation of Peripheral Blood Stem Cells as Compared With Bone Marrow From HLA-Identical Siblings in Adult Patients With Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia. J Clin Oncol. 2002;20:4655–64.
Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158:46–61.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67.
Garnett C, Apperley JF, Pavlů J. Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol. 2013;4:366–78.
Akpek G, Zahurak ML, Piantadosi S, Margolis J, Doherty J, Davidson R, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood 2001;97:1219–26.
Akpek G, Lee SM, Anders V, Vogelsang GB. A high-dose pulse steroid regimen for controlling active chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2001;7:495–502.
Olivieri A, Cimminiello M, Corradini P, Mordini N, Fedele R, Selleri C, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013;122:4111–8.
Olivieri A, Locatelli F, Zecca M, Sanna A, Cimminiello M, Raimondi R, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 2009;114:709–18.
Zhang J, Chen F, Ueki T, Date H. Imatinib for sclerodermatous graft-versus-host disease in lung transplantation. Interact Cardiovasc Thorac Surg. 2015;21:260–2.
Magro L, Mohty M, Catteau B, Coiteux V, Chevallier P, Terriou L, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 2009;114:719–22.
Alsuliman T, Magro L, Coiteux V, Gauthier J, Srour M, Lionet A, et al. The concurrent administration of imatinib with extracorporeal photopheresis leads to complete and durable responses in patients with refractory sclerotic type chronic graft-versus-host disease. Curr Res Transl Med. 2020;68:71–6.
Sánchez-Ortega I, Servitje O, Arnan M, Ortí G, Peralta T, Manresa F, et al. Dasatinib as Salvage Therapy for Steroid Refractory and Imatinib Resistant or Intolerant Sclerotic Chronic Graft-versus-Host Disease. Biol Blood Marrow Transpl. 2012;18:318–23.
Sánchez-Ortega I, Parody R, Servitje O, Muniesa C, Arnan M, Patińo B, et al. Imatinib and dasatinib as salvage therapy for sclerotic chronic graft-vs-host disease. Croat Med J. 2016;57:247–54.
NIH Chronic GVHD Consensus Project [Internet]. [cited 2022 Mar 8]. https://www.astct.org/archive/practice-resources/nih-chronic-gvhd-consensus-project.
Olivieri A, Mancini G, Olivieri J, Marinelli Busilacchi E, Cimminiello M, Pascale SP, et al. Nilotinib in steroid-refractory cGVHD: prospective parallel evaluation of response, according to NIH criteria and exploratory response criteria (GITMO criteria). Bone Marrow Transpl. 2020;55:2077–86.
Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Disco. 2010;9:956–70.
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl Protein-Tyrosine Kinase Inhibitor STI571 Inhibits In Vitro Signal Transduction Mediated by c-Kit and Platelet-Derived Growth Factor Receptors. J Pharm Exp Ther. 2000;295:139–45.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000;96:925–32.
Knight B, Tirnitz–Parker JEE, Olynyk JK. C-kit Inhibition by Imatinib Mesylate Attenuates Progenitor Cell Expansion and Inhibits Liver Tumor Formation in Mice. Gastroenterology 2008;135:969–979.e1.
Schildhaus HU, Cavlar T, Binot E, Büttner R, Wardelmann E, Merkelbach-Bruse S. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008;216:176–82.
Baird K, Comis LE, Joe GO, Steinberg SM, Hakim FT, Rose JJ, et al. Imatinib mesylate for the treatment of steroid-refractory sclerotic-type cutaneous chronic graft-versus-host disease. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:1083–90.
Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41.
Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK, et al. Profound Inhibition of Antigen-Specific T-Cell Effector Functions by Dasatinib. Clin Cancer Res. 2008;14:2484–91.
Cwynarski K, Laylor R, Macchiarulo E, Goldman J, Lombardi G, Melo JV, et al. Imatinib inhibits the activation and proliferation of normal T lymphocytes in vitro. Leukemia 2004;18:1332–9.
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol. 2008;36:1297–308.
Seggewiss R, Loré K, Greiner E, Magnusson MK, Price DA, Douek DC, et al. Imatinib inhibits T-cell receptor–mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005;105:2473–9.
Breccia M, Cannella L, Stefanizzi C, Carotti A, Santopietro M, Alimena G. Efficacy of dasatinib in a chronic myeloid leukemia patient with disease molecular relapse and chronic GVHD after haploidentical BMT: an immunomodulatory effect? Bone Marrow Transpl. 2009;44:331–2.
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, et al. Effects of nilotinib on regulatory T cells: the dose matters. Mol Cancer. 2010;9:22.
Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA Mutations in Gastrointestinal Stromal Tumors: Frequency, Spectrum and In Vitro Sensitivity to Imatinib. J Clin Oncol. 2005;23:5357–64.
Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci USA. 2009;106:1542–7.
van der Wagen L, Janssen J, Raijmakers R, Petersen E, de Witte M, de Jong N, et al. Tyrosine kinase inhibitor levels matter in treating chronic GVHD. Bone Marrow Transpl. 2019;54:1141–4.
Chen GL, Carpenter PA, Broady R, Gregory TK, Johnston LJ, Storer BE, et al. Anti–Platelet-Derived Growth Factor Receptor Alpha Chain Antibodies Predict for Response to Nilotinib in Steroid-Refractory or -Dependent Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transpl. 2018;24:373–80.
Author information
Authors and Affiliations
Contributions
IYA, LM, TA, and MS have contributed to the project conceptualization. TA and MS have contributed equally to this paper preparation and drafting. IYA have supervised the project and contributed to the finalization of the paper. JL have accomplished the statistical analysis. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
TA received honorarium from Biotest France SAS, out of the current paper. ED received honorarium from SOBI board, out of the current paper. MS, GG, SF, JL, PT, PC, has no competing interests. IYA, LM, VA, EF, CEB, YB, received honorarium from NOVARTIS. This trial received an unrestricted fund from NOVARTIS.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srour, M., Alsuliman, T., Labreuche, J. et al. Nilotinib efficacy and safety as salvage treatment following imatinib intolerance and/or inefficacy in steroid refractory chronic graft-versus-host-disease (SR-cGVHD): a prospective, multicenter, phase II study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant 58, 401–406 (2023). https://doi.org/10.1038/s41409-022-01898-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01898-x