Abstract
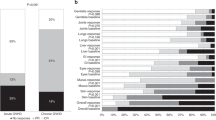
Acute graft versus host disease (aGVHD) is a complication of allogeneic hematopoietic stem cell transplant (HCT) and is associated with significant morbidity and mortality. Steroid refractory aGVHD (SR-aGVHD) carries a particularly grim prognosis. Ruxolitinib has shown promise for treatment of SR-aGVHD in a phase 3 trial; however, safety and efficacy data outside of the clinical trial setting is lacking. We performed a multicenter retrospective study to examine the response to ruxolitinib and its efficacy in patients with SR-aGVHD. We included 59 patients treated with ruxolitinib for SR-aGVHD between 2015 and 2022. Of these 59 patients, 36 patients (61.0%) achieved a complete (CR) or partial response (PR) at 28 days, while 31 patients (52.5%) obtained a CR/PR at day 56. Patients that achieved a CR or PR at day 28 had a higher rate of overall survival (OS; 69.2%), compared with patients that did not (31.6%; p = 0.037). OS at 12 months was 41.5%, with a median OS duration of 5.3 months. Failure free survival (FFS) at 12 months was 29.1%, with a median FFS of 2.6 months. Overall, this real-world experience data support ruxolitinib as the standard of care for SR-aGVHD in a non-controlled trial population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The anonymized datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Zeiser R, Blazar BR. Acute graft-versus-host disease — Biologic process, prevention, and therapy. N. Engl J Med. 2017;377:2167–79.
MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–7.
Tarantino G, Saraceni F, Mancini G, Poiani M, Maroni L, Goteri G, et al. Gastrointestinal complications after allogeneic hematopoietic stem cell transplant: a multidisciplinary approach with early endoscopic evaluation. Clin Hematol Int. 2021;3:161.
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2012;18:1150–63.
Jamani K, Russell JA, Daly A, Stewart D, Savoie L, Duggan P, et al. Prognosis of grade 3–4 acute GVHD continues to be dismal. Bone Marrow Transpl. 2013;48:1359–61.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: Comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94.
Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:1504–18.
Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–60.
Kelly K, Rasko JEJ. Mesenchymal stromal cells for the treatment of graft versus host disease. Front Immunol. 2021;12:761616.
Greinix HT, Ayuk F, Zeiser R. Extracorporeal photopheresis in acute and chronic steroid-refractory graft-versus-host disease: an evolving treatment landscape. Leukemia 2022;36:2558–66.
Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham LC, et al. FDA Approval Summary: Ruxolitinib for Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease. Oncologist. 2020;25:e328–34.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-refractory acute graft-versus-host disease. N. Engl J Med. 2020;382:1800–10.
Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42.
Teshima T. JAK inhibitors: A home run for GVHD patients? Blood. 2014;123:3691–3.
Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135:1739–49.
Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3:e674–89.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. CTCAE versión 5.0. Evaluación de la gravedad de los eventos adversos dermatológicos de las terapias antineoplásicas. Actas Dermosifiliogr. 2021;112:90–2.
Khoury HJ, Wang T, Hemmer MT, Couriel D, Alousi A, Cutler C, et al. Improved survival after acute graft- versus -host disease diagnosis in the modern era. Haematologica. 2017;102:958–66.
Chao N. Finally, a successful randomized trial for GVHD. N. Engl J Med. 2020;382:1853–4.
Zhang Myun, Zhao P, Zhang Y, Wang Jshi. Efficacy and safety of ruxolitinib for steroid-refractory graft-versus-host disease: Systematic review and meta-analysis of randomised and non-randomised studies. PLoS One. 2022;17:e0271979.
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015;29:2062–8.
Shapiro RM, Antin JH. Therapeutic options for steroid-refractory acute and chronic GVHD: an evolving landscape. Expert Rev Hematol. 2020;13:519–32.
MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NKC, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–6.
Perales MA, Ishill N, Lomazow WA, Weinstock DM, Papadopoulos EB, Dastigir H, et al. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transpl. 2007;40:481–6.
Acknowledgements
The study was supported by Novartis and from Princess Margaret Cancer Foundation.
Author information
Authors and Affiliations
Contributions
AM and SML performed data collection, analysis, and manuscript writing. KJ and DK designed the study, provided all administrative support, supervised the project and manuscript revision. All the other authors contributed to data collection, reviewed the manuscript and approved it.
Corresponding author
Ethics declarations
Competing interests
DK received research grant and honoraria from Novartis, Pfizer and Paladin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murray, A., Linn, S.M., Yu, B. et al. Real-world experience with ruxolitinib therapy for steroid-refractory acute graft versus host disease. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02249-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02249-8