Abstract
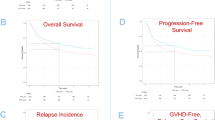
In this registry-based study which includes acute myeloid leukemia patients who underwent a matched unrelated donor allogeneic peripheral-blood stem cell transplantation in complete remission and received post-transplant cyclophosphamide (PTCY) as graft-versus-host disease (GvHD) prophylaxis, we compared 421 recipients without anti-thymocyte globulin (ATG) with 151 patients with ATG. The only significant differences between PTCY and PTCY + ATG cohorts were the median year of transplant and the follow-up period (2017 vs 2015 and 19.6 vs 31.1 months, respectively, p < 0.0001). Overall, 2-year survival was 69.9% vs 67.1% in PTCY and PTCY + ATG, respectively, with deaths related to relapse (39% vs 43.5%), infection (21.9% vs 23.9%) or GvHD (17.1% vs 17.4%) not differing between groups. On univariate comparison, a significantly lower rate of extensive chronic GvHD was found when ATG was added (9.9% vs 21%, p = 0.029), a finding which was not confirmed in the multivariate analysis. The Cox-model showed no difference between PTCY + ATG and PTCY alone with respect to acute and chronic GvHD of all grades, non-relapse mortality, relapse, leukemia-free survival, overall survival, and GvHD-free-relapse-free survival between study cohorts. Our results highlight that the addition of ATG in PTCY does not provide any extra benefit in terms of further GvHD reduction, better GRFS or better survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
AS, ML, BS, AN, and MM had full access to all the data in the study (available upon data-specific request).
References
Zeiser R, Blazar BR. Acute Graft-versus-Host Disease — Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167–79.
Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377:2565–79.
Spyridonidis A. How much immunosuppression do we need? Blood. 2017;129:1241–3.
Chang Y-J, Wu D-P, Lai Y-R, Liu Q-F, Sun Y-Q, Hu J, et al. Antithymocyte Globulin for Matched Sibling Donor Transplantation in Patients With Hematologic Malignancies: A Multicenter, Open-Label, Randomized Controlled Study. J Clin Oncol J Am Soc Clin Oncol. 2020;38:3367–76.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Bonifazi F, Rubio M-T, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transpl. 2020;55:1093–102.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53.
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–9.
Brissot E. Post-transplantation cyclophosphamide versus antithymocyte globulin after ric regimen allo-hct: first analysis of a prospective randomized multicenter trial in recipients of 10/10 matched donors. https://ebmt2021.abstractserver.com/program/#/details/presentations/1316 (accessed Apr 2021).
Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol OncolJ Hematol Oncol. 2020;13:87.
Cytryn S, Abdul-Hay M. Haploidentical Hematopoietic Stem Cell Transplantation Followed by ‘Post-Cyclophosphamide’: The Future of Allogeneic Stem. Cell Transplant Clin Hematol Int. 2020;2:49–58.
Bashey A, Zhang M-J, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol J Am Soc Clin Oncol. 2017;35:3002–9.
Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124:1428–37.
El-Cheikh J, Devillier R, Dulery R, Massoud R, Al Chami F, Ghaoui N, et al. Impact of Adding Antithymocyte Globulin to Posttransplantation Cyclophosphamide in Haploidentical Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2020;20:617–23.
Makanga DR, Guillaume T, Willem C, Legrand N, Gagne K, Cesbron A, et al. Posttransplant Cyclophosphamide and Antithymocyte Globulin versus Posttransplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis for Peripheral Blood Stem Cell Haploidentical Transplants: Comparison of T Cell and NK Effector Reconstitution. J Immunol Balt Md 1950. 2020;205:1441–8.
Wang Y, Wu D-P, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol OncolJ Hematol Oncol. 2019;12:88.
Kanate AS, Nagler A, Savani B. Summary of Scientific and Statistical Methods, Study Endpoints and Definitions for Observational and Registry-Based Studies in Hematopoietic Cell Transplantation. Clin Hematol Int. 2019;2:2–4.
Nunes NS, Kanakry CG. Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front Immunol. 2019;10. https://doi.org/10.3389/fimmu.2019.02668.
Alanazi W, Chen S, Lipton JH, Kim DD, Viswabandya A, Kumar R, et al. Post-Transplant Cyclophosphamide Combined with Anti-Thymocyte Globulin as Graft-versus-Host Disease Prophylaxis for Allogeneic Hematopoietic Cell Transplantation in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Acta Haematol. 2021;144:66–73.
Kunacheewa C, Owattanapanish W, Jirabanditsakul C, Issaragrisil S. Post-Transplant Cyclophosphamide and Thymoglobulin, a Graft-Versus-Host Disease Prophylaxis in Matched Sibling Donor Peripheral Blood Stem. Cell Transpl Cell Transpl. 2020;29:963689720965900.
Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral blood stem cell transplants. Eur J Haematol. 2019;102:486–93.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol OncolJ Hematol Oncol. 2018;11:40.
Salas MQ, Chen S, Lam W, Pasic I, Gerbitz A, Michelis FV, et al. Less Is More: Superior Graft-versus-Host Disease-Free/Relapse-Free Survival with Reduced-Intensity Conditioning and Dual T Cell Depletion in Acute Myelogenous Leukemia. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2020;26:1511–9.
Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137:3291–305.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e143.
De Jong CN, Meijer E, Bakunina K, Nur E, van Marwijk Kooij M, de Groot MR, et al. Post-Transplantation Cyclophosphamide after Allogeneic Hematopoietic Stem Cell Transplantation: Results of the Prospective Randomized HOVON-96 Trial in Recipients of Matched Related and Unrelated Donors. Blood. 2019;134:1–1.
Alousi AM, Brammer JE, Saliba RM, Andersson B, Popat U, Hosing C, et al. Phase II Trial of Graft-versus-Host Disease Prophylaxis with Post-Transplantation Cyclophosphamide after Reduced-Intensity Busulfan/Fludarabine Conditioning for Hematological Malignancies. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:906–12.
Bradstock KF, Bilmon I, Kwan J, Micklethwaite K, Blyth E, Deren S, et al. Single-Agent High-Dose Cyclophosphamide for Graft-versus-Host Disease Prophylaxis in Human Leukocyte Antigen-Matched Reduced-Intensity Peripheral Blood Stem Cell Transplantation Results in an Unacceptably High Rate of Severe Acute Graft-versus-Host Disease. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:941–4.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167.
Baron F, Labopin M, Blaise D, Lopez-Corral L, Vigouroux S, Craddock C, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:389–96.
Nagler A, Dholaria B, Labopin M, Socie G, Huynh A, Itälä-Remes M, et al. The impact of anti-thymocyte globulin on the outcomes of Patients with AML with or without measurable residual disease at the time of allogeneic hematopoietic cell transplantation. Leukemia. 2020;34:1144–53.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TCG, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2:e194–203.
Acknowledgements
We thank the ALWP-EBMT staff for help with data management. The study was accomplished thanks to the contributing centers of the EBMT registry which provided patient data; a complete list appears in the Supplementary Appendix.
Author information
Authors and Affiliations
Contributions
AS, ML, and MM designed the study; ML performed the statistical analyses; AS wrote the paper; BS, AN, and MM revised the paper; EB, IM, JC, GC, FC, JV, PR, MR, EM, HLW, DB, GG, NK, YK, SG, AB, BS, AN, MM were the principal investigators at the centers recruiting the largest numbers of patients for the study. All authors reviewed the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board at each site and complied with country-specific regulatory requirements. The study was conducted in accordance with the declaration of the Helsinki and Good Clinical Practice guidelines. Patients provide informed consent authorizing the use of their personal information for research purposes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spyridonidis, A., Labopin, M., Brissot, E. et al. Should anti-thymocyte globulin be added in post-transplant cyclophosphamide based matched unrelated donor peripheral blood stem cell transplantation for acute myeloid leukemia? A study on behalf of the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 57, 1774–1780 (2022). https://doi.org/10.1038/s41409-022-01816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01816-1
This article is cited by
-
Post-transplant cyclophosphamide at 80 mg/kg with low dose post-engraftment anti-thymocyte globulin in haploidentical transplantation with myeloablative conditioning
Bone Marrow Transplantation (2024)
-
Posttransplant cyclophosphamide beyond haploidentical transplantation
Annals of Hematology (2023)