Abstract
There is an increased risk of GVHD and of non-relapse mortality (NRM) after allogeneic stem cell transplantations (alloSCT) when mismatched unrelated donors (MMUD) are used. In Europe, it is standard practice to use rabbit anti-thymocyte globulin (rATG) to reduce the high NRM and GVHD risks after MMUD alloSCT. As an alternative to rATG, post-transplantation Cyclophosphamide (PTCy) is in increasing clinical use. It is currently impossible to give general recommendations regarding preference for one method over another since comparative evidence from larger data sets is lacking. To improve the evidence base, we analyzed the outcome of rATG vs. PTCy prophylaxis in adult patients with hematologic malignancies undergoing first peripheral blood alloSCT from MMUD (9/10 antigen match) between Jan 2018 and June 2021 in the database of the European Society for Blood and Marrow Transplantation (EBMT). We performed multivariate analyses using the Cox proportional-hazards regression model. We included 2123 patients in the final analyses (PTCy, n = 583; rATG, n = 1540). p values and hazard ratios (HR) presented here are multivariate outcomes. Two years after alloSCT we found a lower NRM in the PTCy group of 18% vs. 24.9% in the rATG group; p = 0.028, HR 0.74. Overall survival in the PTCy cohort was higher with 65.7% vs. 55.7% in the rATG cohort; p < 0.001, HR 0.77. Progression-free survival was also better in the PTCy patients with 59.1% vs. 48.8% when using rATG; p = 0.001, 0.78. The incidences of chronic GVHD and acute GVHD were not significantly different between the groups. We found significantly lower NRM as well as higher survival in recipients of peripheral blood alloSCTs from MMUD receiving PTCy as compared to rATG. The results of the current analysis suggest an added value of PTCy as GVHD prophylaxis in MMUD alloSCT.
Similar content being viewed by others
Introduction
One of the main clinical challenges of allogeneic stem cell transplantation (alloSCT) is its inherent non-relapse mortality (NRM) with graft-versus-host disease (GVHD) as a major contributing factor. This problem is aggravated when mismatched unrelated stem cell donors (MMUD) are used, leading to specifically high NRM [1].
There is consensus in the field that patients after MMUD alloSCT should get an intensive GVHD prophylaxis regimen. It has been standard of care to use rabbit anti-thymocyte globulin (rATG, also termed anti-T-cell globulin or anti-T-lymphocyte globulin; products: Grafalon® or Thymoglobulin®) in alloSCTs from MMUD in Europe to decrease the GVHD and NRM risks. However, the prevention strategies of GVHD are currently changing. Cyclophosphamide given after alloSCT (post-transplant Cyclophosphamide, PTCy) is another option, which is increasingly used in some alloSCT centers.
Currently it is not possible to make sound evidence based decisions on the use of rATG or PTCy in MMUD alloSCT since comparative data from large data sets is missing. In addition, no randomized trials specifically compared PTCy vs. rATG prophylaxis in MMUD alloSCT. Previous smaller studies gave inconsistent results. A European Society for Blood and Marrow Transplantation (EBMT) matched control study suggested that PTCy could have advantages over rATG in the MMUD setting [2]. In the CTN 1703 and CTN 1203 randomized trials [3, 4], as well as in a comparative retrospective study [5], which showed benefit for PTCy, only a few (7/8) MMUD patients were enrolled. Two retrospective studies and a meta-analysis dedicated to MMUD alloSCT showed no significant reduction in the incidence or severity of aGVHD or cGVHD, in patients receiving PTCy, while a decreasing rate was estimated after adjusting for propensity [6,7,8]. The meta-analysis [7] highlighted a reduced NRM in the PTCy arm as compared to the rATG arm, which is in line with the results of the propensity-adjusted retrospective study [5]. However, in an EBMT retrospective cohort, the GVHD-free, relapse-free survival was not significantly different between PTCy and rATG [8]. Taken together the available evidence base is insufficient for clinical decision making.
To improve the evidence base, we analyzed outcomes of rATG vs. PTCy prophylaxis in adult patients with hematologic malignancies undergoing first peripheral blood alloSCT from 9/10 antigen MMUD between Jan 2018 and June 2021 in the database of the EBMT.
Subjects and methods
Study design and data collection
This is a retrospective multicenter analysis using the data set of the EBMT registry. The EBMT is a voluntary working group of more than 600 transplant centers which are required to report regular follow up on all consecutive stem cell transplantations. Audits are routinely performed to determine the accuracy of the data. The study was planned and approved by the Transplant Complications Working Party of the EBMT. All patients gave their written informed consent to use their personal information for research purposes. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Eligibility criteria for this analysis included patients older than 18 years of age at alloSCT with hematologic malignancies (acute lymphoblastic leukemia, acute myeloid leukemia, lymphoma, chronic lymphocytic leukemia, myelodysplastic syndrome or myeloproliferative neoplasms), who underwent a first alloSCT from a 9/10 antigen mismatched unrelated donor (MMUD), from a peripheral blood stem cells source, between Jan 2018 and June 2021 in the database of the EBMT. Only patients receiving either rATG or PTCy based GVHD prophylaxis (without a combined use of both) were included. Additionally, patients with more than one previous autologous transplantation, ex vivo T-cell depletion, or use of Alemtuzumab (Campath) were not included in the study. Data collected included recipient and donor characteristics (age, sex, cytomegalovirus serostatus and Karnofsky performance status score), diagnosis and status at transplant and transplant-related factors, including conditioning regimen, stem cell source and GVHD prophylaxis. GVHD grading was performed according to published criteria for acute GVHD [9] and chronic GVHD [10]. For the purpose of this study, all necessary data were collected according to the EBMT guidelines, using the EBMT Minimum Essential Data forms.
Statistical analysis
Median values and interquartile ranges (IQR), and minimum and maximum values were used to describe quantitative variables; frequency and percentage were used for categorical variables. Main patient-, disease-, and transplant-related characteristics were compared using Pearson’s chi-squared or Fisher test for categorical variables, and the Kruskal–Wallis rank sum test for quantitative variables between the two groups.
Study endpoints were non-relapse mortality (NRM), overall survival (OS), progression-free survival (PFS), relapse incidence (RI), GVHD-free/relapse-free survival (GRFS), and incidence and severity of acute GVHD and chronic GVHD. The initial time was the date of transplant for all endpoints. NRM was defined as death without relapse/progression, PFS was defined as survival without relapse or progression, RI was defined as disease recurrence, GRFS was defined as survival without incidence of relapse, or grade III–IV acute GVHD, or severe chronic GVHD. Probabilities of OS, PFS and GRFS were calculated using the Kaplan-Meier method. Cumulative incidence was used to estimate NRM, RI, as well as acute and chronic GVHD in a competing risk setting, where death and relapse were considered as competing risks as appropriate [11]. Multivariate analyses were performed using the Cox cause-specific proportional-hazards model for all end points. All known potential risk factors, and variables differing significantly across the groups were included in the multivariate models: patient age at transplant, year of transplant, patient and donor gender, donor to patient CMV combination, Disease Risk Index (DRI), Karnofsky Performance Status (KPS), any level of total body irradiation (TBI), conditioning intensity (RIC vs. MAC). Center effect was taken into account by introducing a random effect or ‘frailty’ into all models. Results were expressed as the hazard ratio (HR) with the 95% confidence interval (95% CI). All tests were two-sided with a type 1 error rate fixed at 0.05. Statistical analyses were performed with R 4.3.0 software (R Development Core Team, Vienna, Austria) packages.
Results
The baseline characteristics of the study population are presented in Table 1. A total of 2123 patients were included, from which 1540 (73%) received rATG, and 583 (27%) received PTCy as a GVHD prophylaxis.
Overall, the majority of patients was transplanted for acute leukemia (58.6%), myelodysplastic syndrome (MDS)/ myeloproliferative neoplasm (MPN) (25.6%) or lymphoma (15.1%). A high proportion of patients had a low/intermediate Disease Risk Index (DRI, 72%), and myeloablative conditioning (MAC) was more frequently performed (53.9%) than reduced intensity conditioning (RIC).
Patients in the rATG group were older, with a median age of 56.1 years (IQR 44.7, 63.8) vs. 51.7 years in the PTCy group (IQR 40.0, 62.2) (p ≤ 0.001), with a similar proportion of males (58% in rATG vs. 60% in PTCy, p = 0.48), and less recent transplants (p < 0.01), along with a significantly lower use of TBI (14.3% vs. 20.4%, p < 0.01). The remaining parameters were balanced between the two groups.
Median follow up was 2.0 years (95% CI [2–2.2]) in the PTCy arm and 2.4 years (95% CI [2.2–2.6]) in the rATG arm.
Survival, RI and NRM
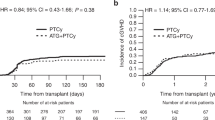
Univariate outcomes are shown in Fig. 1 and Table 2. The results of the multivariate analyses are summarized in Table 3.
Patients receiving PTCy had a significantly lower NRM as compared to patients receiving rATG (2 years incidence: 18% vs. 24.9%; HR: 0.74 [95% CI 0.56–0.97], p = 0.028). Similarly, OS and PFS showed a statistically significant and clinically meaningful benefit for PTCy arm, with a higher OS (2 years incidence: 65.7% vs. 55.7%; HR: 0.77 [95% CI 0.65–0.90], p < 0.001), and a higher PFS (2 years incidence: 59.1% vs. 48.8%; HR: 0.78 [95% CI 0.67–0.91], p = 0.001). RI did not differ significantly between the two groups, however, there was a trend toward a lower relapse rate in the PTCy arm vs. the rATG arm (2 years incidence: 22.9% vs. 26.2%; HR: 0.82 [95% CI 0.67–1.01], p = 0.068) (see Fig. 1).
Relapse of the underlying malignancy was the most frequent cause of death, accounting for 287 (44%) of total deaths in both arms, followed by NRM causes: infections (161 [19%]), GVHD (150 [18%]) and other alloSCT-related causes (150 [9%]) of total deaths. Secondary malignancies contributed to ~1% of total deaths, proportion for each arm are presented in Table 4.
After conducting an additional multivariate cox analysis, adjusting for HLA mismatches location additionally to the previous factors, we found no hazard ratio (HR) for HLA mismatches to be statistically significant. This indicates that the differences in outcomes are not attributable to HLA-A, B, or C mismatches in our context. Furthermore, our results consistently demonstrate a significant benefit in favor of PTCy relatively to ATG. Specifically, we observed a notable reduction in non-relapse mortality (NRM) associated with PTCy compared to ATG, with a hazard ratio (HR) of 0.66 [CI 95%: (0.49–0.88); p value: 0.005], similarly there was a significant improvement in overall survival (OS) for PTCy relatively to ATG [HR: 0.69, CI 95%: (0.56–0.83); p value < 0.001], we also observed a reduced risk for progression-free survival (PFS) in PTCy relatively to ATG [HR: 0.71 CI 95%: (0.59–0.86); p value < 0.001]. These results were further supported by the fact that no significant interaction term between PTCy vs. ATG and Mismatch Location has been found, suggesting that the beneficial effect of PTCy over ATG is consistent across different HLA mismatch subgroups.
Incidence of acute and chronic GVHD, and GRFS
No significant difference was observed in acute and chronic GVHD outcomes between the two groups (see Fig. 2). The incidence of acute GVHD grades II–IV in patients receiving PTCy vs. rATG (100 days incidence: 29.9% vs. 32.5%; HR: 0.83 [95% CI 0.66–1.04], p = 0.11), and the incidence of severe acute GVHD grades III–IV (100 days incidence: 11.4% vs. 13.8%; HR: 0.78 [95% CI 0.59–1.05], p = 0.1), showed no significant difference. Similarly, overall chronic GVHD for patients receiving PTCy vs. rATG (2 years incidence: 31.7% vs. 30.3%; HR 0.95 [95% CI 0.74–1.22], p = 0.67), as well as extensive chronic GVHD (2 years incidence: 12.7% vs. 14.2%; HR 0.83 [95% CI 0.63–1.10], p = 0.20) were not statistically different.
GRFS was significantly higher in the PTCy arm compared to the rATG arm (2 years incidence: 46% vs. 35.3%; HR: 0.80 [95% 0.68–0.94], p = 0.006) (see Fig. 1).
Discussion
In MMUD alloSCT, rATG or PTCy are readily used as part of the GVHD prophylaxis strategy. In Europe, it has been standard of care to use rATG in alloSCTs with a high GVHD risk [12], but there is to date no direct evidence available on PTCy prophylaxis vs. rATG use. Only a few (7/8) MMUD patients were enrolled in the CTN 1703 and CTN 1203 randomized trials [3, 4], as well as in a comparative retrospective study, suggesting efficacy of PTCy in the MMUD setting [5].
The results from the current study add to this emerging evidence by showing better outcomes of PTCy vs. rATG regarding NRM and survival after MMUD alloSCT in our real-world retrospective dataset. These data add to previous evidence comparing PTCy vs. rATG prophylaxis in MMUD alloSCT. One study found in patients with lymphoproliferative diseases undergoing 9/10 MMUD alloSCT a significantly lower extensive cGVHD rate when PTCy was used (PTCy 5% vs. ATG 18%) [13]. Another publication shows reduced aGVHD rates in MMUD alloSCT recipients when PTCy is used vs. ATG [14]. Jiminez et al. found Improved GRFS after PTCy vs. ATG-based MMUD alloSCT [15]. One smaller retrospective study demonstrated lower aGVHD and NRM rates in the PTCy arm without significant association to cGVHD [16]. Two retrospective studies and a meta-analysis showed no significant reduction in the incidence or severity of aGVHD or cGVHD after PTCy vs. rATG use in MMUD alloSCT, while a decreasing rate was estimated after adjusting for propensity [7, 8, 17]. A meta-analysis [7] highlighted a reduced NRM in the PTCy arm vs. rATG, win line with the results of the propensity-adjusted retrospective study [5] and with our current results. However, in another EBMT dataset report describing a smaller population of patients with lymphoma as underlying disease, GVHD-free, relapse-free survival was not significantly improved in a previous report from the EBMT [8].
The limitations of our current study are inherent to all retrospective real-world datasets, with low granularity, risk of underreporting and potential confounding factors. We also noted significant differences in baseline characteristics, with the rATG group being slightly older at diagnosis and transplantation, and having received more radiation therapy. The amount of missing data however was surprisingly low. Furthermore, because of the advent of PTCY prophylaxis is a relatively recent practice change, the observation times are still relatively limited, precluding conclusions regarding long-term outcome and the occurrence of late effects. For instance, we did not observe differences in secondary malignancies but long-term follow up will be needed to answer the question if PTCy has relevant carcinogen effects in this specific setting. We also noticed a wide variety of immunosuppressive regimens given alongside the rATG or PTCY prophylaxis, whose effect is, by design, difficult to tease out.
In the present study, we found a statistically non-significant trend toward a lower incidence of relapse in patients receiving PTCy vs. rATG. These data raises the question of whether patients with certain tumor entities benefit particularly strongly from PTCy use. Future studies will need to focus on the differential impact of PTCy vs. rATG on relapse rates in different tumor entities (e.g., lymphoid malignancies vs. myeloid neoplasms) led by disease specific working parties with access to large sets of patient data (e.g., EBMT or CIBMTR).
Taking together all the available evidence from the current study as well as from previous publications, it becomes evident that rATG and PTCy are both of clinical use in MMUD alloSCT. One of the possible next steps is to investigate the combination of both strategies to further increase efficacy in the MMUD setting [18]. A combination of rATG and PTCy has been tested by several investigators in haploidentical SCT (haploSCT) [19,20,21]. Gao et al. combined PTCy and rATG with tacrolimus in a single arm study in 67 haploSCT recipients and found a low incidence of severe acute GVHD [20]. Chen at al. compared the outcome after rATG/PTCy (n = 61) with historical data from patients undergoing haploSCT with sirolimus/PT-Cy prophylaxis and found similar aGVHD and cGVHD rates in both arms but a higher overall survival in the rATG/PTCy arm [19]. Zhang et al. published a randomized controlled trial where 122 haploSCT recipients were randomly assigned 1:1 to either a PTCy/ATG or a standard-dose ATG group (“Beijing Protocol”, ATG: 10 mg/kg) [21]. The cumulative incidence of grade II–IV acute GVHD was significantly lower in the PTCy/ATG group (11.5% vs. 39.3%). Furthermore, 2-year overall survival (75.4% vs. 54.1%) and disease-free survival (72.7% vs. 55.0%) were significantly improved in the PTCy/ATG group. In the setting of MMUD there is less data available on the combination of PTCy and ATG. Deotare et al. published their experience with a combination of PTCy and rATG in MUD (n = 22) and MMUD (n = 6) alloSCT comparing it to with 27 historical cohort patients receiving rATG [22]. The cumulative incidence of acute GVHD (17% vs. 33%) and severe grade III–IV aGVHD (7% vs. 25%) was significantly lower in the rATG/PTCy cohort but survival was not different. Xue et al. reported on a pilot study in 21 alloSCT recipients including 4 MMUD alloSCT where they added low dose rATG to their standard PTCy GVHD prophylaxis [23]. Using a matched-pair analysis with a historic control receiving PTCy only, they found significantly lower cGVHD incidence (15% vs. 41%) but no significant differences in aGVHD and in survival outcomes. In summary, there is currently not enough evidence to recommend a combination of rATG with PTCy in routine clinical use in MMUD alloSCT but considerable emerging data suggesting that this should be a focus area for clinical research.
In this study, we found significantly lower NRM as well as higher survival in recipients of peripheral blood alloSCTs from MMUD receiving PTCy compared to rATG. The results of the current analysis build on the available evidence suggesting a preferential use of PTCy as GVHD prophylaxis in MMUD alloSCT.
Data availability
Individual participant data will not be shared because patients agreed to data sharing with EBMT as well as with publication of results, but not to share data with third parties.
References
Saraceni F, Labopin M, Gorin NC, Blaise D, Tabrizi R, Volin L, et al. Matched and mismatched unrelated donor compared to autologous stem cell transplantation for acute myeloid leukemia in first complete remission: a retrospective, propensity score-weighted analysis from the ALWP of the EBMT. J Hematol Oncol. 2016;9:79. https://doi.org/10.1186/s13045-016-0314-x.
Battipaglia G, Labopin M, Kroger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–9. https://doi.org/10.1182/blood.2019000487.
Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48. https://doi.org/10.1056/NEJMoa2215943.
Bolanos-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43. https://doi.org/10.1016/S2352-3026(18)30221-7.
Maurer K, Ho VT, Inyang E, Cutler CS, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. 2023. https://doi.org/10.1182/bloodadvances.2023009791.
Modi B, Hernandez-Henderson M, Yang D, Klein J, Dadwal S, Kopp E, et al. Ruxolitinib as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2019;25:265–9. https://doi.org/10.1016/j.bbmt.2018.09.003.
Mushtaq MU, Shahzad M, Tariq E, Iqbal Q, Chaudhary SG, Zafar MU, et al. Outcomes with mismatched unrelated donor allogeneic hematopoietic stem cell transplantation in adults: a systematic review and meta-analysis. Front Oncol. 2022;12:1005042. https://doi.org/10.3389/fonc.2022.1005042.
Paviglianiti A, Mussetti A, Ngoya M, Boumendil A, Fegueux N, Bonifazi F, Gulbas Z, et al. A comparison between ATG and PT-CY graft-versus-host-disease prophylaxis in patients with lymphoma undergoing reduced intensity conditioning regimen HSCT from 1 antigen MMUD. In Bone Marrow Transplant. vol. 57. London, N1 9XW, England: Springernature, 2022. pp. 224–225.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. https://doi.org/10.1016/j.bbmt.2014.12.001.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67. https://doi.org/10.1016/S2352-3026(19)30256-X.
Paviglianiti A, Ngoya M, Pena M, Boumendil A, Gulbas Z, Ciceri F, et al. Graft-versus-host-disease prophylaxis with ATG or PTCY in patients with lymphoproliferative disorders undergoing reduced intensity conditioning regimen HCT from one antigen mismatched unrelated donor. Bone Marrow Transplant. 2024. https://doi.org/10.1038/s41409-024-02225-2.
Dybko J, Sobczyk-Kruszelnicka M, Makuch S, Agrawal S, Dudek K, Giebel S, et al. The benefits of the post-transplant cyclophosphamide in both haploidentical and mismatched unrelated donor setting in allogeneic stem cells transplantation. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms24065764.
Jimenez Jimenez A, Komanduri K, Brown S, Wang T, Pereira D, Goodman M, et al. Improved GRFS after posttransplant cyclophosphamide-based vs ATG-based HLA-mismatched unrelated donor transplant. Blood Adv. 2022;6:4491–500.
Nykolyszyn C, Granata A, Pagliardini T, Castagna L, Harbi S, Bouabdallah R, et al. Posttransplantation cyclophosphamide vs. antithymocyte globulin as GVHD prophylaxis for mismatched unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:349–55. https://doi.org/10.1038/s41409-019-0682-2.
Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant cyclophosphamide versus thymoglobulin in HLA-mismatched unrelated donor transplant for acute myelogenous leukemia and myelodysplastic syndrome. Transplant Cell Ther. 2021;27:760–7. https://doi.org/10.1016/j.jtct.2021.06.018.
Dulery R, Brissot E, Mohty M. Combining post-transplant cyclophosphamide with antithymocyte globulin for graft-versus-host disease prophylaxis in hematological malignancies. Blood Rev. 2023:101080. https://doi.org/10.1016/j.blre.2023.101080.
Chen TT, Lin CC, Lo WJ, Hsieh CY, Lien MY, Lin CH, et al. Antithymocyte globulin plus post-transplant cyclophosphamide combination as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation for hematological malignancies. Int J Hematol. 2022;115:525–33. https://doi.org/10.1007/s12185-021-03280-x.
Gao WH, Zhu JY, Wang LN, Wan M, Wang L, Devillier R, et al. Post-transplantation cyclophosphamide combined with tacrolimus and low-dose post-engraftment anti-thymoglobulin as GVHD prophylaxis for patients undergoing peripheral blood stem cell transplantation from haploidentical family donor: a single center analysis. Front Med. 2023;10:1140217. https://doi.org/10.3389/fmed.2023.1140217.
Zhang W, Gui R, Zu Y, Zhang B, Li Z, Zhang Y, et al. Reduced-dose post-transplant cyclophosphamide plus low-dose post-transplant anti-thymocyte globulin as graft-versus-host disease prophylaxis with fludarabine-busulfan-cytarabine conditioning in haploidentical peripheral blood stem cell transplantation: a multicentre, randomized controlled clinical trial. Br J Haematol. 2023;200:210–21. https://doi.org/10.1111/bjh.18483.
Deotare U, Atenafu EG, Loach D, Michelis FV, Kim DD, Thyagu S, et al. Reduction of severe acute graft-versus-host disease using a combination of pre transplant anti-thymocyte globulin and post-transplant cyclophosphamide in matched unrelated donor transplantation. Bone Marrow Transplant. 2018;53:361–5. https://doi.org/10.1038/s41409-017-0053-9.
Xue E, Lorentino F, Lupo Stanghellini MT, Giglio F, Piemontese S, Clerici DT, et al. Addition of a single low dose of anti T-lymphocyte globulin to post-transplant cyclophosphamide after allogeneic hematopoietic stem cell transplant: a pilot study. J Clin Med. 2022;11. https://doi.org/10.3390/jcm11041106.
Funding
The authors thank the following funding agencies for supporting this work: José Carreras Leukämie-Stiftung (3R/2019, 23R/2021), Deutsche Krebshilfe (70113519), Deutsche Forschungsgemeinschaft (PE 1450/7-1, PE 1450/9-1) and Stiftung Charité BIH (BIH_PRO_549, Focus Group Vascular Biomedicine). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CP performed statistical analyses; OP and ZP designed the study and wrote the manuscript. All authors performed research read, edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
OP has received honoraria or travel support from Gilead, Jazz, MSD, Novartis, Pfizer and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi and SOBI. HS reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, all paid to her institution. She has also received non-financial support from Gilead, Pfizer, the EBMT (European Society for Blood and Marrow transplantation) and the CIBMTR (Center for International Bone Marrow Transplantation Research). IM received honoraria or travel support from Novartis, Sanofi, SOBI, Takeda. CCL received honoraria or travel support from Gilead/Kite and Therakos. Consulting fees for advisory board from Gilead/Kite, Nektar Therapeutics. FB participated to AB and received speaker fees from NEOVII and SANOFI. PD reports consultancy for AbbVie, AstraZeneca, Beigene, BMS, Gilead, Miltenyi, Novartis, Riemser; speakers bureau for AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Novartis, Riemser, Roche; research support from Riemser (all to institution).
Ethical approval
The study was approved by the EBMT review board. Patients had to sign an informed consent document that permitted sharing of clinical data according to national rules.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Penack, O., Abouqateb, M., Peczynski, C. et al. PTCy versus ATG as graft-versus-host disease prophylaxis in mismatched unrelated stem cell transplantation. Blood Cancer J. 14, 45 (2024). https://doi.org/10.1038/s41408-024-01032-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-024-01032-8