Abstract
Monoclonal antibodies against checkpoint receptors or its ligands have demonstrated high response rates and durable remissions in patients with relapsed Hodgkin lymphoma (HL) and other lymphoid malignancies. However, most patients will eventually progress on therapy and may benefit from further treatments including allogenic hematopoietic cell transplantation (allo-HCT). Furthermore, the use of checkpoint inhibitors (CPI) has emerged as a treatment option for patients relapsing after allo-HCT. The immune effects of the checkpoint blockade leading to a T-cell activation have raised some concerns on the safety of these therapies used either before or after allo-HCT, due to the potential risk of graft-versus-host disease (GVHD). Furthermore, CPI might also induce other immune toxicities, that can affect almost any organ, as a result of the dysregulation on the immune system balance. This review aims to focus on the evidence behind the use of CPI in patients with lymphoma who undergo allo-HCT. We summarize the clinical data generated to date about the use of CPI in HL and other lymphoid malignancies, the mechanisms of checkpoint inhibition in the context of allo-HCT as well as the clinical and biological observations of different GVHD prophylaxis in this setting. Furthermore, we discuss the evidence from retrospective series and early clinical trials on the feasibility and safety of the use of CPI in patients who relapsed after allo-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. https://doi.org/10.1056/NEJMoa1411087.
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–53. https://doi.org/10.1056/NEJMoa1601202.
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2018;36:1428–39. https://doi.org/10.1200/JCO.2017.76.0793.
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–94. https://doi.org/10.1016/S1470-2045(16)30167-X.
Bekoz H, Karadurmus N, Paydas S, Turker A, Toptas T, Firatli Tuglular T, et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol: Off J Eur Soc Med Oncol. 2017;28:2496–502. https://doi.org/10.1093/annonc/mdx341.
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–7.
Holderried TAW, Fraccaroli A, Schumacher M, Heine A, Brossart P, Stelljes M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin’s Lymphoma. Bone Marrow Transplant. 2019;54:1662–7. https://doi.org/10.1038/s41409-019-0498-0.
Merryman RW, Kim HT, Zinzani PL, Carlo-Stella C, Ansell SM, Perales MA, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129:1380–8. https://doi.org/10.1182/blood-2016-09-738385.
Schoch LK, Cooke KR, Wagner-Johnston ND, Gojo I, Swinnen LJ, Imus P, et al. Immune checkpoint inhibitors as a bridge to allogeneic transplantation with posttransplant cyclophosphamide. Blood Adv. 2018;2:2226–9. https://doi.org/10.1182/bloodadvances.2018019208.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–86. https://doi.org/10.1158/2159-8290.CD-18-0367.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. https://doi.org/10.1016/j.cell.2017.01.017.
Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H, et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2020;497:178–89. https://doi.org/10.1016/j.canlet.2020.10.024.
Onishi H, Fujimura A, Oyama Y, Yamasaki A, Imaizumi A, Kawamoto M, et al. Hedgehog signaling regulates PDL-1 expression in cancer cells to induce anti-tumor activity by activated lymphocytes. Cell Immunol. 2016;310:199–204. https://doi.org/10.1016/j.cellimm.2016.08.003.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. https://doi.org/10.1016/j.ccell.2015.03.001.
Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like Protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–47. e312. https://doi.org/10.1016/j.cell.2018.11.010.
Xie M, Huang X, Ye X, Qian W. Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma. Int Immunopharmacol. 2019;77:105999. https://doi.org/10.1016/j.intimp.2019.105999.
Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–20. https://doi.org/10.1038/nrclinonc.2016.168.
Brodska B, Otevrelova P, Salek C, Fuchs O, Gasova Z, Kuzelova K. High PD-L1 expression predicts for worse outcome of leukemia patients with concomitant NPM1 and FLT3 mutations. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20112823.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. https://doi.org/10.1084/jem.192.7.1027.
Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6. https://doi.org/10.1126/sciadv.abd2712.
Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall survival and long-term safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33:2004–12. https://doi.org/10.1200/JCO.2014.58.3708.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. https://doi.org/10.1056/NEJMoa1501824.
Scapin G, Yang X, Prosise WW, McCoy M, Reichert P, Johnston JM, et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 2015;22:953–8. https://doi.org/10.1038/nsmb.3129.
Wang M, Wang J, Wang R, Jiao S, Wang S, Zhang J, et al. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun Biol. 2019;2:392. https://doi.org/10.1038/s42003-019-0642-9.
Tan S, Zhang H, Chai Y, Song H, Tong Z, Wang Q, et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun. 2017;8:14369. https://doi.org/10.1038/ncomms14369.
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–39. https://doi.org/10.1056/NEJMoa1917346.
Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–33.e17. https://doi.org/10.1016/j.cell.2017.07.024.
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. https://doi.org/10.1126/science.1202947.
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. https://doi.org/10.1126/science.1160062.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. https://doi.org/10.1056/NEJMoa1003466.
Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134:1144–53. https://doi.org/10.1182/blood.2019000324.
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the efficacy and safety of Pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35:2125–32. https://doi.org/10.1200/JCO.2016.72.1316.
Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief report: cancer immunotherapy in patients with preexisting rheumatic disease: the Mayo Clinic experience. Arthritis Rheumatol. 2018;70:356–60. https://doi.org/10.1002/art.40397.
Singh M, Jackson KJL, Wang JJ, Schofield P, Field MA, Koppstein D, et al. Lymphoma driver mutations in the pathogenic evolution of an iconic human autoantibody. Cell. 2020;180:878–94. e819. https://doi.org/10.1016/j.cell.2020.01.029.
Diefenbach CS, Hong F, Ambinder RF, Cohen JB, Robertson MJ, David KA, et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020;7:e660–70. https://doi.org/10.1016/S2352-3026(20)30221-0.
Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131:1183–94. https://doi.org/10.1182/blood-2017-10-811224.
Moskowitz AJ, Advani RH, Bartlett NL, Vose JM, Ramchandren R, Feldman TA. et al. Brentuximab Vedotin and Nivolumab for relapsed or refractory classic Hodgkin lymphoma: long-term follow-up results from the Single-Arm Phase 1/2 Study. Blood. 2019;134 Suppl_1:238–238. https://doi.org/10.1182/blood-2019-122576 .
Ramchandren R, Domingo-Domenech E, Rueda A, Trneny M, Feldman TA, Lee HJ, et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the Phase II CheckMate 205 Study. J Clin Oncol: Off J Am Soc Clin Oncol. 2019;37:1997–2007. https://doi.org/10.1200/JCO.19.00315.
Bröckelmann PJ, Goergen H, Keller U, Meissner J, Ordemann R, Halbsguth TV. et al. Nivolumab and AVD for early-stage unfavorable Hodgkin lymphoma (NIVAHL). Blood. 2019;134 Suppl_1:236–236. https://doi.org/10.1182/blood-2019-122406.
Yasenchak CA, Bordoni R, Yazbeck V, Patel-Donnelly D, Anderson T, Larson T. et al. Phase 2 Study of frontline Brentuximab Vedotin Plus Nivolumab in patients with Hodgkin lymphoma aged ≥60 years. Blood. 2019;134 Suppl_1:237. https://doi.org/10.1182/blood-2019-124866.
Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2019;37:3291–9. https://doi.org/10.1200/JCO.19.01389.
Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a Phase Ib Study. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34:2698–704. https://doi.org/10.1200/JCO.2015.65.9789.
Armand P, Janssens AM, Gritti G, Radford J, Timmerman JM, Pinto A, et al. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood. 2020. https://doi.org/10.1182/blood.2019004753.
Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a Single-Arm, Phase II Study. J Clin Oncol: Off J Am Soc Clin Oncol. 2019;37:481–9. https://doi.org/10.1200/JCO.18.00766.
Ansell S, Gutierrez ME, Shipp MA, Gladstone D, Moskowitz A, Borello I, et al. A Phase 1 Study of Nivolumab in combination with Ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood. 2016;128:183. https://doi.org/10.1182/blood.V128.22.183.183.
Jain N, Ferrajoli A, Basu S, Thompson PA, Burger JA, Kadia TM. et al. A Phase II Trial of Nivolumab combined with Ibrutinib for patients with Richter transformation. Blood. 2018;132 Suppl_1:296–296. https://doi.org/10.1182/blood-2018-99-120355.
Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–78. https://doi.org/10.1016/S2352-3026(18)30217-5.
Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–206. https://doi.org/10.1200/JCO.2012.48.3685.
De Goycoechea D, Stalder G, Martins F, Duchosal MA. Immune checkpoint inhibition in classical Hodgkin lymphoma: from early achievements towards new perspectives. J Oncol. 2019;2019:9513701. https://doi.org/10.1155/2019/9513701.
Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner HH, et al. Hodgkin lymphoma. Nat Rev Dis Prim. 2020;6:61. https://doi.org/10.1038/s41572-020-0189-6.
Paul S, Zahurak M, Luznik L, Ambinder RF, Fuchs EJ, Bolanos-Meade J, et al. Non-myeloablative allogeneic transplantation with post-transplant cyclophosphamide after immune checkpoint inhibition for classic Hodgkin lymphoma: a Retrospective Cohort Study. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2020;26:1679–88. https://doi.org/10.1016/j.bbmt.2020.06.012.
Desnoyer A, Broutin S, Delahousse J, Maritaz C, Blondel L, Mir O, et al. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: part 2, immune checkpoint inhibitor antibodies. Eur J Cancer. 2020;128:119–28. https://doi.org/10.1016/j.ejca.2020.01.003.
Nieto JC, Roldan E, Jimenez I, Fox L, Carabia J, Orti G, et al. Posttransplant cyclophosphamide after allogeneic hematopoietic cell transplantation mitigates the immune activation induced by previous nivolumab therapy. Leukemia. 2020. https://doi.org/10.1038/s41375-020-0851-8.
Martinez C, Carpio C, Heras I, Rios-Herranz E, Buch J, Gutierrez A, et al. Potential survival benefit for patients receiving allogeneic hematopoietic stem cell transplantation after nivolumab therapy for relapse/refractory Hodgkin lymphoma: real-life experience in Spain. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2020;26:1534–42. https://doi.org/10.1016/j.bbmt.2020.02.003.
Rivas MM, Berro M, Prates MV, Yantorno S, Fiad L, Arbelbide JA, et al. Allogeneic stem cell transplantation improves survival in relapsed Hodgkin lymphoma patients achieving complete remission after salvage treatment. Bone Marrow Transplant. 2020;55:117–25. https://doi.org/10.1038/s41409-019-0640-z.
Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study—a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–7. https://doi.org/10.3324/haematol.2011.045757.
Manson G, Herbaux C, Brice P, Bouabdallah K, Stamatoullas A, Schiano JM, et al. Prolonged remissions after anti-PD-1 discontinuation in patients with Hodgkin lymphoma. Blood. 2018;131:2856–9. https://doi.org/10.1182/blood-2018-03-841262.
Dada R, Usman B. Allogeneic hematopoietic stem cell transplantation in r/r Hodgkin lymphoma after treatment with checkpoint inhibitors: feasibility and safety. Eur J Haematol. 2019;102:150–6. https://doi.org/10.1111/ejh.13186.
Ikegawa S, Meguri Y, Kondo T, Sugiura H, Sando Y, Nakamura M, et al. PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade. Blood Adv. 2019;3:4081–94. https://doi.org/10.1182/bloodadvances.2019000134.
Ito A, Kim SW, Matsuoka KI, Kawakita T, Tanaka T, Inamoto Y, et al. Safety and efficacy of anti-programmed cell death-1 monoclonal antibodies before and after allogeneic hematopoietic cell transplantation for relapsed or refractory Hodgkin lymphoma: a multicenter retrospective study. Int J Hematol. 2020;112:674–89. https://doi.org/10.1007/s12185-020-02960-4.
El Cheikh J, Massoud R, Abudalle I, Haffar B, Mahfouz R, Kharfan-Dabaja MA, et al. Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant. 2017;52:1074–7. https://doi.org/10.1038/bmt.2017.69.
Herbaux C, Merryman R, Devine S, Armand P, Houot R, Morschhauser F, et al. Recommendations for managing PD-1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: taming a necessary evil. Blood. 2018;132:9–16. https://doi.org/10.1182/blood-2018-02-811174.
Angenendt L, Schliemann C, Lutz M, Rebber E, Schulze AB, Weckesser M, et al. Nivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:443–5. https://doi.org/10.1038/bmt.2015.266.
Aslan A, Aras T, Ozdemir E. Successful treatment of relapsed/refractory Hodgkins lymphoma with nivolumab in a heavily pretreated patient with progressive disease after both autologous and allogeneic stem cell transplantation. Leuk Lymphoma. 2017;58:754–5. https://doi.org/10.1080/10428194.2016.1213835.
Godfrey J, Bishop MR, Syed S, Hyjek E, Kline J. PD-1 blockade induces remissions in relapsed classical Hodgkin lymphoma following allogeneic hematopoietic stem cell transplantation. J Immunother Cancer. 2017;5:11. https://doi.org/10.1186/s40425-017-0211-z.
McDuffee E, Aue G, Cook L, Ramos-Delgado C, Shalabi R, Worthy T, et al. Tumor regression concomitant with steroid-refractory GvHD highlights the pitfalls of PD-1 blockade following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:759–61. https://doi.org/10.1038/bmt.2016.346.
Singh AK, Porrata LF, Aljitawi O, Lin T, Shune L, Ganguly S, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin’s lymphoma. Bone Marrow Transplant. 2016;51:1268–70. https://doi.org/10.1038/bmt.2016.111.
Villasboas JC, Ansell SM, Witzig TE. Targeting the PD-1 pathway in patients with relapsed classic Hodgkin lymphoma following allogeneic stem cell transplant is safe and effective. Oncotarget. 2016;7:13260–4. https://doi.org/10.18632/oncotarget.7177.
Yared JA, Hardy N, Singh Z, Hajj S, Badros AZ, Kocoglu M, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:850–2. https://doi.org/10.1038/bmt.2015.346.
Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129:2471–8. https://doi.org/10.1182/blood-2016-11-749556.
Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221–8. https://doi.org/10.1182/blood-2017-01-761346.
Schoch LK, Borrello I, Fuchs EJ, Bolanos-Meade J, Huo JS, Gojo I, et al. Checkpoint inhibitor therapy and graft versus host disease in allogeneic bone marrow transplant recipients of haploidentical and matched products with post-transplant cyclophosphamide. Blood. 2016;128:4571–4571. https://doi.org/10.1182/blood.V128.22.4571.4571.
Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood. 2020;135:2182–91. https://doi.org/10.1182/blood.2019004710.
Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–8. https://doi.org/10.1182/blood-2008-07-168468.
Khouri IF, Fernandez Curbelo I, Turturro F, Jabbour EJ, Milton DR, Bassett RL Jr, et al. Ipilimumab plus Lenalidomide after allogeneic and autologous stem cell transplantation for patients with lymphoid malignancies. Clin Cancer Res: Off J Am Assoc Cancer Res. 2018;24:1011–8. https://doi.org/10.1158/1078-0432.CCR-17-2777.
Acknowledgements
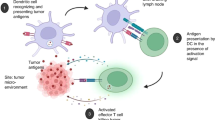
Figure 1 was created with BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SB has received honoraria from Roche, BMS, and Janssen, not related with the present study. PB declares having received honoraria from Amgen, BMS, Gilead, Novartis, and Pfizer and not related with the present article. PB has received funding from Asociación Española Contra el Cancer. Ideas Semilla 2019. The other author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bobillo, S., Nieto, J.C. & Barba, P. Use of checkpoint inhibitors in patients with lymphoid malignancies receiving allogeneic cell transplantation: a review. Bone Marrow Transplant 56, 1784–1793 (2021). https://doi.org/10.1038/s41409-021-01268-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01268-z
This article is cited by
-
Posttransplant cyclophosphamide beyond haploidentical transplantation
Annals of Hematology (2023)
-
Allogeneic hematopoietic stem cell transplantation for NK/T-cell lymphoma: an international collaborative analysis
Leukemia (2023)
-
Immune checkpoint inhibitors and allogeneic transplant in lymphoid malignancies: a deceptive friend story
Bone Marrow Transplantation (2021)