Abstract
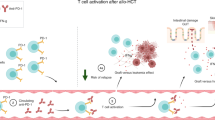
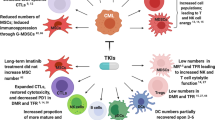
The immune system plays a major role in preventing infections and cancers. Impairment in immunity may facilitate the development of neoplasia owing to defective immune surveillance, among other mechanisms. Immune evasion plays a significant role in relapse after allogeneic hematopoietic cell transplantation (alloHCT); one purported mechanism is through immune checkpoint signaling pathways. Checkpoint inhibitors (CPIs) are FDA approved for relapsed classical Hodgkin’s Lymphoma (cHL), primary mediastinal large B cell Lymphoma (PMBCL) and other solid tumors. Retrospective studies evaluating the outcomes of alloHCT after prior exposure to CPIs showed favorable survival outcomes but high rates of graft-versus-host disease (GVHD); the risk appears to be lower when using post-transplant cyclophosphamide as GVHD prophylaxis. CPIs have increasingly been used to prevent or treat post-alloHCT relapse. Available data, albeit limited, supports the clinical activity of CPIs in post-alloHCT relapse; however, serious and even fatal cases of GVHD have been reported. The optimal timing, schedule, dosing, and patients likely to benefit from this strategy are yet to be identified. In this review, we highlight the immune system’s role in cancer surveillance and relapse prevention and discuss the current clinical evidence of CPIs use in post-alloHCT relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N. Engl J Med. 1957;257:491–6.
Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and biology of relapse after stem cell transplantation. Bone Marrow Transplant. 2018;53:1379–89.
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–7.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48.
Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9.
Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–92.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84.
Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–76.
Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46.
Chohan K, Ansell SM. Current salvage therapies in Hodgkin lymphoma. Leuk Lymphoma. 2022;63:1267–80.
Castagna L, Santoro A, Carlo-Stella C. Salvage Therapy for Hodgkin’s Lymphoma: A Review of Current Regimens and Outcomes. J Blood Med. 2020;11:389–403.
Jacoby MA, Duncavage EJ, Chang GS, Miller CA, Shao J, Elliott K, et al. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight. 2018;3:e98962.
Quek L, Ferguson P, Metzner M, Ahmed I, Kennedy A, Garnett C, et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016;1:193–204.
Cairo MS, Jordan CT, Maley CC, Chao C, Melnick A, Armstrong SA, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biological Considerations of Hematological Relapse following Allogeneic Stem Cell Transplantation Unrelated to Graft-versus-Tumor Effects: State of the Science. Biol Blood Marrow Transplant. 2010;16:709–28.
Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25:603–11.
Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N Engl J Med. 2018;379:2330–41.
Zeiser R, Vago L. Mechanisms of immune escape after allogeneic hematopoietic cell transplantation. Blood. 2019;133:1290–7.
Norde WJ, Maas F, Hobo W, Korman A, Quigley M, Kester MG, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71:5111–22.
Hutten TJA, Norde WJ, Woestenenk R, Wang RC, Maas F, Kester M, et al. Increased Coexpression of PD-1, TIGIT, and KLRG-1 on Tumor-Reactive CD8(+) T Cells During Relapse after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2018;24:666–77.
Manfredi F, Noviello M, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Exhausted Central Memory and Memory Stem T Cells Specific for Leukemia Infiltrate the Bone Marrow of AML Patients Relapsing after Allogeneic HSCT. Blood. 2018;132:2028.
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36:1428–39.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N Engl J Med. 2014;372:311–9.
Moskowitz CH, Zinzani PL, Fanale MA, Armand P, Johnson NA, Radford JA, et al. Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Primary End Point Analysis of the Phase 2 Keynote-087 Study. Blood. 2016;128:1107.
Merryman RW, Kim HT, Zinzani PL, Carlo-Stella C, Ansell SM, Perales MA, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129:1380–8.
Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-Intensity Conditioning Compared With Conventional Allogeneic Stem-Cell Transplantation in Relapsed or Refractory Hodgkin’s Lymphoma: An Analysis From the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62.
Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–47.
Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transpl. 2008;14:418–25.
Merryman RW, Castagna L, Giordano L, Ho VT, Corradini P, Guidetti A, et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia. 2021;35:2672–83.
Perales MA, Awan F, Boumendil A, Chen S, Bazarbachi A, Finel A, et al. Outcomes of allogeneic HCT in patients with Hodgkin lymphoma in the era of checkpoint inhibitors: a joint CIBMTR and EBMT analysis. Abstract from the 48th annual EBMT meeting. Bone Marrow Transpl. 2022;57:11–5.
Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J Clin Oncol. 2019;37:3291–9.
Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study. J Clin Oncol. 2019;37:3081–9.
Oran B, Garcia-Manero G, Saliba RM, Alfayez M, Al-Atrash G, Ciurea SO, et al. Posttransplantation cyclophosphamide improves transplantation outcomes in patients with AML/MDS who are treated with checkpoint inhibitors. Cancer. 2020;126:2193–205.
Tschernia NP, Kumar V, Moore DT, Vincent BG, Coombs CC, Van Deventer H, et al. Safety and Efficacy of Pembrolizumab Prior to Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia. Transpl Cell Ther. 2021;27:1021.e1–5.
Nieto JC, Roldán E, Jiménez I, Fox L, Carabia J, Ortí G, et al. Posttransplant cyclophosphamide after allogeneic hematopoietic cell transplantation mitigates the immune activation induced by previous nivolumab therapy. Leukemia. 2020;34:3420–5.
Herbaux C, Merryman R, Devine S, Armand P, Houot R, Morschhauser F, et al. Recommendations for managing PD-1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: taming a necessary evil. Blood. 2018;132:9–16.
Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing Roles of CD28:B7 and CTLA-4:B7 Pathways in Regulating In Vivo Alloresponses in Murine Recipients of MHC Disparate T Cells. J Immunol. 1999;162:6368–77.
Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–8.
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375:143–53.
Khouri IF, Fernandez Curbelo I, Turturro F, Jabbour EJ, Milton DR, Bassett RL Jr, et al. Ipilimumab plus Lenalidomide after Allogeneic and Autologous Stem Cell Transplantation for Patients with Lymphoid Malignancies. Clin Cancer Res. 2018;24:1011–8.
Angenendt L, Schliemann C, Lutz M, Rebber E, Schulze AB, Weckesser M, et al. Nivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:443–5.
Yared JA, Hardy N, Singh Z, Hajj S, Badros AZ, Kocoglu M, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:850–2.
Aslan A, Aras T, Özdemir E. Successful treatment of relapsed/refractory Hodgkins lymphoma with nivolumab in a heavily pretreated patient with progressive disease after both autologous and allogeneic stem cell transplantation. Leuk Lymphoma. 2017;58:754–5.
Mori S, Ahmed W, Patel RD, Dohrer AL. Steroid Refractory Acute Liver GVHD in a Hodgkin’s Patient after Allogeneic Stem Transplant Cell Transplantation Following Treatment with Anti PD-1 Antibody, Nivolumab, for Relapsed Disease. Biol Blood Marrow Transplant. 2016;22:S392–S3.
Singh AK, Porrata LF, Aljitawi O, Lin T, Shune L, Ganguly S, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin’s lymphoma. Bone Marrow Transplant. 2016;51:1268–70.
De la Hoz A, Foolad F, Gallegos C, Kornblau S, Kontoyiannis DP. Nivolumab–induced encephalitis post allogeneic stem cell transplant in a patient with Hodgkin’s disease. Bone Marrow Transplant. 2019;54:749–51.
Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129:2471–8.
Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. PD-1 blockade for relapsed lymphoma post–allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221–8.
Herrera AF, Burton C, Radford J, Miall F, Townsend W, Santoro A, et al. Avelumab in relapsed/refractory classical Hodgkin lymphoma: phase 1b results from the JAVELIN Hodgkins trial. Blood Adv. 2021;5:3387–96.
Holderried TAW, Fraccaroli A, Schumacher M, Heine A, Brossart P, Stelljes M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin’s Lymphoma. Bone Marrow Transpl. 2019;54:1662–7.
Tang Y, Zhou Z, Yan H, You Y. Case Report: Preemptive Treatment With Low-Dose PD-1 Blockade and Azacitidine for Molecular Relapsed Acute Myeloid Leukemia With RUNX1-RUNX1T1 After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2022;13:810284.
Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood. 2020;135:2182–91.
Wang AY, Kline J, Stock W, Kosuri S, Artz A, Larson RA, et al. Unexpected Toxicities When Nivolumab Was Given as Maintenance Therapy following Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2020;26:1025–7.
Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–45.
Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–606.
Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant. 2000;26:769–74.
Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transpl. 2014;20:1785–90.
Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl. 2015;21:454–9.
Chen Y-H, Zhang X, Cheng Y-F, Chen H, Mo X-D, Yan C-H, et al. Long-term follow-up of CD19 chimeric antigen receptor T-cell therapy for relapsed/refractory acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Cytotherapy. 2020;22:755–61.
Zhang C, Wang X-Q, Zhang R-L, Liu F, Wang Y, Yan Z-L, et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia. 2021;35:1563–70.
Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol. 2021;96:671–9.
Hua J, Zhang J, Wu X, Zhou L, Bao X, Han Y, et al. Allogeneic Donor-Derived Anti-CD19 CAR T Cell Is a Promising Therapy for Relapsed/Refractory B-ALL After Allogeneic Hematopoietic Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2020;20:610–6.
Brudno JN, Somerville RPT, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016;34:1112–21.
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Investig. 2016;126:3363–76.
Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4:e1027469.
Ding L, Wang Y, Hong R, Zhao H, Zhou L, Wei G, et al. Efficacy and Safety of Chimeric Antigen Receptor T Cells in Acute Lymphoblastic Leukemia With Post-Transplant Relapse. Front Oncol. 2021;11:750218.
Liu P, Liu M, Lyu C, Lu W, Cui R, Wang J, et al. Acute Graft-Versus-Host Disease After Humanized Anti-CD19-CAR T Therapy in Relapsed B-ALL Patients After Allogeneic Hematopoietic Stem Cell Transplant. Front Oncol. 2020;10:573822.
Jain T, Sauter CS, Shah GL, Maloy MA, Chan J, Scordo M, et al. Safety and feasibility of chimeric antigen receptor T cell therapy after allogeneic hematopoietic cell transplantation in relapsed/ refractory B cell non-Hodgkin lymphoma. Leukemia. 2019;33:2540–4.
Lutfi F, Holtzman N, Siglin J, Bukhari A, Mustafa Ali M, Kim D, et al. Chimeric antigen receptor T-cell therapy after allogeneic stem cell transplant for relapsed/refractory large B-cell lymphoma. Br J Haematol. 2021;192:212–6.
Schubert M-L, Dietrich S, Stilgenbauer S, Schmitt A, Pavel P, Kunz A, et al. Feasibility and Safety of CD19 Chimeric Antigen Receptor T Cell Treatment for B Cell Lymphoma Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2020;26:1575–80.
Chen X, Li X, Liu Y, Zhang Z, Zhang X, Huang J, et al. A Phase I clinical trial of chimeric antigen receptor-modified T cells in patients with relapsed and refractory lymphoma. Immunotherapy. 2020;12:681–96.
Alkhaldi H, Sewell D, Ning Y, Kallen ME, Emadi A, Hardy NM, et al. Durable response to ivosidenib in post-transplant relapse and leukemic transformation of myelodysplastic syndrome with new complex karyotype and IDH1 R132C mutation. Leuk Lymphoma. 2022;63:3000–3.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med. 2018;378:2386–98.
Stein EM. Enasidenib, a targeted inhibitor of mutant IDH2 proteins for treatment of relapsed or refractory acute myeloid leukemia. Fut Oncol. 2018;14:23–40.
Author information
Authors and Affiliations
Contributions
HA wrote the first draft. MK-D, RE, and MA provided expert review of the literature relevant to the topic and reviewed and edited the paper. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alkhaldi, H., Kharfan-Dabaja, M., El Fakih, R. et al. Safety and efficacy of immune checkpoint inhibitors after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 58, 1075–1083 (2023). https://doi.org/10.1038/s41409-023-02073-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02073-6